Histochemical Fiber Types
Basic Concept - Cell Differentiation
The formation of muscle fibers from mesodermal
cells through a series of transitional cell types (premyoblast,
myoblast and myotube or secondary fiber) is a classical example
of cellular differentiation. Cellular differentiation leads to an efficient
and mutually advantageous division of labor among the tissues and organs
of the body.
In skeletal muscles, differentiation continues after the fibers
have been formed and have reached a functional state.
Physiological differentiation follows cellular differentiation, and
creates populations of fast and slow fibers with appropriate sources of
energy for contraction,
either aerobic (using blood-borne oxygen for complete oxidation of
substrates)
or anaerobic (incomplete oxidation of carbohydrates without need
for oxygen).
Red and White Muscle
Certain muscles of the carcass are particularly dark or red. This color
difference is caused by a red pigment, myoglobin, in the sarcoplasm
(cytoplasm) of muscle fibers.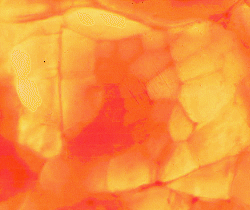
Hemoglobin, the pigment of red blood cells, brings oxygen
to capillaries on the muscle fiber surface.
From here, the transport of oxygen to the interior of the fiber is facilitated
by myoglobin. Thus, fibers specialized for aerobic metabolism develop a
high myoglobin concentration.
The dominant work of some muscles is to maintain a standing posture
or to contract slowly during locomotion, chewing or breathing. Such muscles
tend to contain a high proportion of slow-contracting and fatigue-resistant
fibers with a high myoglobin concentration. The capillary bed of red muscles
is more dense than in white muscles.
Way back in 1873, the great French histologist Ranvier had already found
that dark red muscles
-
(1) contract slowly,
-
(2) develop tetanus (lock in full contraction) at lower rates of stimulation,
-
(3) have relatively more sarcoplasm,
-
(4) have more distinct
longitudinal striations, and
-
(5) are more resistant to fatigue.
Don't get longitudinal striations mixed up with In
transverse sections of muscle fibers, differences in myofibrillar size,
in the regularity of myofibrillar arrangement, and in the degree of myofibrillar
separation may create two distinct patterns named by German histologists,
felderstruktur in slow fibers and fibrillenstruktur in fast
fibers.
For
every generalization, we can expect an underlying complexity of exceptions!
A detailed explanation is available elsewhere.
Fast and Slow Fibers
At first sight, historically speaking, it appeared that the relationship
between fast and slow fibers in meat animals was quite simple. From the
time of Ranvier onwards, it had been known that fast fibers were usually
white, while slow fibers were usually red. When redness was found to be
due to myoglobin, and myoglobin was found to be correlated with aerobic
metabolism, this explained the relationship between redness and speed of
contraction. The pale or white fibers with a low aerobic potential were
found to be well endowed with glycolytic enzymes that enabled them to obtain
energy rapidly by the incomplete oxidation of glycogen.
This explained why white fibers soon became fatigued once their glycogen
stores were depleted and why they had to wait for the removal of lactate
by the circulatory system.
At the extremes of the range in physiological differentiation
(fast white fibers versus slow red fibers) these discoveries are still
valid. The problem, as we see it now, is that there are also fibers
with a fast contraction speed and a dual energy supply.
In other words, some fast fibers have both aerobic and anaerobic capabilities.
The discovery of these fibers coincided in a most confusing way with a
growing awarenesss that slow red fibers in meat animals and poultry were
rather different from those of frogs and other creepy animals so frequently
used in biomedical research. It is difficult to write a research report
on muscle fiber types without giving them names. Unfortunately, everybody
seemed to use different names, and the numbers of fiber types that were
recognized tended to be a function of the number of histochemical techniques
used to identify them. What a bummer.
Cutting a long story short, we may generalize
as follows.
-
Red = beta-R = Type I, distinguished by histochemical features indicative
of a slow contraction speed (eg., acid-stable ATPase, alkali-labile ATPase)
plus features indicative of strong aerobic metabolism (eg., strong mitochondrial
SDH activity).
-
Intermediate = alpha-R = Type II red, distinguished by features indicative
of a fast contraction speed (eg., acid-labile, alkali-stable ATPase) plus
features indicative of strong aerobic metabolism.
-
White = alpha-W = Type II white, distingusihed by features indicative of
a fast contraction speed plus features indicative of weak aerobic metabolism
(eg., low SDH activity).
Here is an example of an ATPase reaction.
A frozen section of muscle is exposed to ATP solution and the ATPase obligingly
cleaves off the phosphate. But the phosphate is invisible and tries to
move around. First we stop it moving by precipitating the phosphate with
cobalt, then we make the cobalt salt go black so we can see where it is
by converting it to a sulfide. If that is all we do, all the fibers may
go black, because they have all got ATPase. So first off all, before we
start the reactions described above, we expose the frozen sections of meat
to solutions (acetic acid, formaldehyde, etc) that will knock out the isoenzyme
in either the fast or slow fibers. Then we can see differences between
fibers, as above. It's a lot more complicated than this in reality, but
hopefully this will help you understand this image!
Here is an example of an SDH reaction.
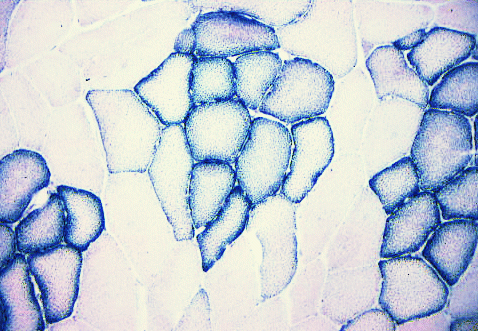
SDH = succinate dehydrogenase, an enzyme specific to mitochondria.
Each little granule of diformazan (the reaction product of nitroblue tetrazolium)
indicates where there are mitochondria.
Here is an example of a phosphorylase reaction.
 Phosphorylase
is the first enzyme involved in glycogenolysis. It normally breaks down
glycogen, but we can trick it into running backwards so that it makes new
glycogen (amylose) that we can stain with iodine. The catch is, that the
reaction works best if there is some natural glycogen present in the muscle
fiber to start the reaction. Thus, absence of a phosphorylase reaction
does not automatically mean that there is no phosphorylase present!
Phosphorylase
is the first enzyme involved in glycogenolysis. It normally breaks down
glycogen, but we can trick it into running backwards so that it makes new
glycogen (amylose) that we can stain with iodine. The catch is, that the
reaction works best if there is some natural glycogen present in the muscle
fiber to start the reaction. Thus, absence of a phosphorylase reaction
does not automatically mean that there is no phosphorylase present!
Here is an example of a stain for triglyceride - Sudan Black B.
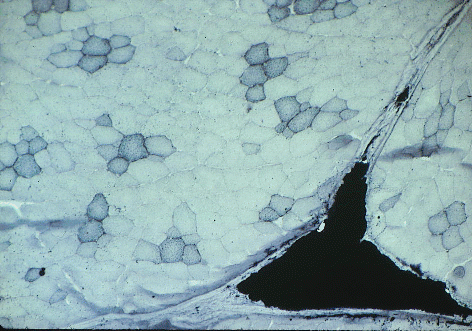 Sudan black has stained the lipid droplets inside
red muscle fibers in this slice of pork, and it has also stained a large
triangle of intramuscular (marbling) adipose cells.
Sudan black has stained the lipid droplets inside
red muscle fibers in this slice of pork, and it has also stained a large
triangle of intramuscular (marbling) adipose cells.
Many of the cellular features associated with aerobic and anaerobic
metabolism in muscle fibers are fairly straightforward. Aerobic fibers
are
-
served by a more dense capillary meshwork than fibers with a poor aerobic
potential;
-
their sarcoplasm contains more mitochondria and more lipid droplets; and
-
the enzymes involved in aerobic metabolism are more concentrated.
Quantitatively, however, the range from aerobic to anaerobic metabolism
is usually a continuous variable and is seldom broken into discontinuous
steps.
From which we may deduce two points:
-
Firstly, by manipulating the pH of ATPase incubation media, it is possible
to generate more than two staining responses, and these do not fit very
well with the categories of histochemical fiber types.
-
Secondly, there is evidence that the physiological differentiation of muscle
fibers is a dynamic balance in the division of labor, and that the balance
may change during growth or in response to a change in the work pattern
of a muscle.
Thus, to some researchers, the histochemical categorization of muscle fibers
by any method, including myofibrillar ATPase, is merely a useful, but artificial
subdivision of a continuously variable range. We (because this is the view
I support) conclude that
muscle fibers undergo a continual alteration throughout life as an adaptation
to changing functional demands, and that "fiber type" merely reflects the
consitution of a fiber at any particular time.
However, click on to another researcher's home page, and you might read
the opposite! From an agricultural viewpoint, this is particularly interesting
since it suggests the existance of some degree of genetic or developmental
plasticity in the fiber type continuum. In meat animals, this might be
a vital link in relating muscle growth to meat quality.
Intracellular differentiation
Physiological differentiation may vary intracellularly
along and across individual muscle fibers, at least as far as aerobic metabolism
is concerned. But as far as is known at present, factors relating to
contraction speed are fairly uniform within individual fibers. Aerobic
metabolism, as indicated by the distribution of mitochondria, may be graduated
radially so that the subsarcolemmal region has a high level of aerobic
metabolism while the central axis has a low level. Mitochondria from peripheral
and axial regions of the muscle fiber may differ in their biochemical characteristics,
and. proportional mitochondrial volume and maximal rate of oxygen consumption
are linearly related among different muscle regions.
The subsarcolemmal concentration of mitochondria in some types of muscle
fibers may be related to the fact that the supply of oxygen to individual
muscle fibers arrives in capillaries that wind over the surface of the
muscle fiber. Mitochondria are larger in red fibers than in intermediate
or white fibers and, in red fibers, they may form thick longitudinal columns
between the myofibrils. The arterial and venous elements of muscle capillaries
tend to occur in an alternating manner along the length of the fiber, with
longer arterial segments of capillaries in white muscle relative to red
muscle.
 This image taken off my research computer
shows the results of automatic mapping of the SDH deposits in a muscle
fiber. Dark blue shows high SDH, and light blue shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying the
radial gradients of SDH activity in different types of meat animals. Gradients
have been measured for pigs,geese,ducks,
and turkeys.
This image taken off my research computer
shows the results of automatic mapping of the SDH deposits in a muscle
fiber. Dark blue shows high SDH, and light blue shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying the
radial gradients of SDH activity in different types of meat animals. Gradients
have been measured for pigs,geese,ducks,
and turkeys.
TROPHIC EFFECT OF NERVES
Motor neurons exert a long term regulation over the physiological and metabolic
properties of the fibers in their motor unit. This is often called the
trophic effect of nerve on muscle. The word trophic implies something of
a nutritive effect, as if the nerve was feeding the muscle, but its current
usage sometimes includes possible non-nutritive effects such as the frequency
patterns of nerve impulses to the muscle. The idea that nerves might have
a trophic function is far from new, and probably originates from ancient
observations on the degenerative fate that overtakes many organs once they
have been denervated.
Trophic effects may be bi-directional since there are some retrograde
trophic effects that travel from the muscle to the nerve. For example,
presynaptic terminal boutons on motor neuron perikarya are lost when axons
are cut, and they are restored when neuromuscular contact is re-established.
Similarly, there are soluble fractions from skeletal muscle that may promote
growth and differentiation in the embryonic spinal cord.
FIBER TYPE CHANGES DURING GROWTH
Histochemical fiber types are important in meat animals because they influence
meat quality. Histochemical fiber types also react differently during the
conversion of muscles to meat, because they contain different levels of
glycogen and anaerobic enzymes. Before it became known that fibers could
change from one type to another, growth-related changes in fiber types
were not adequately controlled in agricultural experiments on muscle fiber
histochemistry.
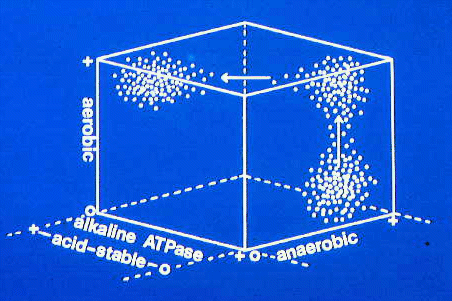
This three dimensional plot shows the sorts of changes that may
occur as fiber type clusters are transformed during muscle growth.
-
PIGS The differences in red coloration between various pork muscles
are related to the incidence of aerobic and anaerobic muscle fibers. An
unusual feature of most pork muscles is that the tendency for aerobic fibers
to be located centrally in their fasciculi is more extreme than in any
other species yet identified. Thus, the concentric arrangement of primary
myotubes and secondary fetal fibers is preserved after birth. The reason
why it is well preserved in pigs yet becomes muddled in other species is
unknown. In the longissimus dorsi muscle, fiber type differentiation on
the basis of aerobic enzyme activity is only slightly developed at birth,
but becomes well developed by 2 weeks. The percentage of white fibers in
pork muscles differs between breeds and is related to the extent to which
the meat yield of a breed has been improved by selective breeding. The
muscles of wild pigs are dominated by red fibers while those of the most
improved breeds are dominated by white fibers with a large diameter. In
Pigs, many muscles show growth-related changes
in the proportions of histochemical fiber types.
-
SHEEP and CATTLEThe concentric fascicular arrangement of fiber types is
difficult to see, and fiber type ratios change during growth.





 Phosphorylase
is the first enzyme involved in glycogenolysis. It normally breaks down
glycogen, but we can trick it into running backwards so that it makes new
glycogen (amylose) that we can stain with iodine. The catch is, that the
reaction works best if there is some natural glycogen present in the muscle
fiber to start the reaction. Thus, absence of a phosphorylase reaction
does not automatically mean that there is no phosphorylase present!
Phosphorylase
is the first enzyme involved in glycogenolysis. It normally breaks down
glycogen, but we can trick it into running backwards so that it makes new
glycogen (amylose) that we can stain with iodine. The catch is, that the
reaction works best if there is some natural glycogen present in the muscle
fiber to start the reaction. Thus, absence of a phosphorylase reaction
does not automatically mean that there is no phosphorylase present!
 Sudan black has stained the lipid droplets inside
red muscle fibers in this slice of pork, and it has also stained a large
triangle of intramuscular (marbling) adipose cells.
Sudan black has stained the lipid droplets inside
red muscle fibers in this slice of pork, and it has also stained a large
triangle of intramuscular (marbling) adipose cells.
 This image taken off my research computer
shows the results of automatic mapping of the SDH deposits in a muscle
fiber. Dark blue shows high SDH, and light blue shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying the
radial gradients of SDH activity in different types of meat animals. Gradients
have been measured for pigs,geese,ducks,
and turkeys.
This image taken off my research computer
shows the results of automatic mapping of the SDH deposits in a muscle
fiber. Dark blue shows high SDH, and light blue shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying the
radial gradients of SDH activity in different types of meat animals. Gradients
have been measured for pigs,geese,ducks,
and turkeys.
