PRENATAL DEVELOPMENT OF SKELETAL MUSCLE
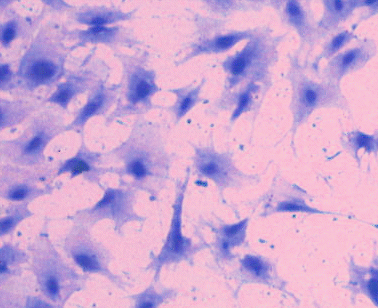 Premyoblasts
Premyoblasts
INTRODUCTION
Since my happy days as a graduate student at the University of Wisconsin
under the supervision of Bob Cassens, I have been convinced that the manipulation
of prenatal development and innervation of muscle offers some tremendous
opportunities for the long-term improvement of meat yield and quality (Ph.D.
Thesis, 1971; Development and Innervation of Muscle Subject to Selective
Breeding). After a decade or so of descriptive histology and histochemistry,
however, it became equally obvious that the tools required for manipulation
primarily would be biochemical and genetic. With a poor grasp of these
subjects, I decided to leave the task to others better qualified than myself
and to redirect my efforts elsewhere (developing methods for the on-line
evaluation of meat quality . However, I have enjoyed keeping pace as
a general reader with the advances made in prenatal muscle development
and innervation, which I will attempt to introduce below (details
and references are available).
SOMITES
In normal development, the prenatal origin of striated skeletal muscles
is from myoblasts. The cells that give rise to myoblasts may be
called premyoblasts or presumptive myoblasts. The origin of premyoblasts
is rather difficult to determine since premyoblasts are difficult to distinguish
morphologically from other types of stem cells destined to give rise to
other types of tissues.
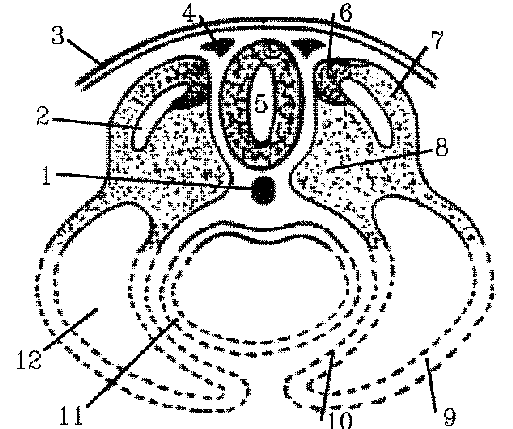 This diagram shows a greatly
simplified plan of a transverse section through an embryo. The bottom of
the figure, in particular, bears little or no resemblence to either mammalian
or avian embryos but is included to indicate the general relationships
of the somites to the endoderm of the future gut (11), to the coelom or
body cavity (12) and to the parietal lateral plate mesoderm or future ventral
body wall (9) that develops later. Other key landmarks are the hollow dorsal
nerve cord (5), notochord (1), and neural crest (4). Each somite is composed
of three zones around the myocoele cavity:
This diagram shows a greatly
simplified plan of a transverse section through an embryo. The bottom of
the figure, in particular, bears little or no resemblence to either mammalian
or avian embryos but is included to indicate the general relationships
of the somites to the endoderm of the future gut (11), to the coelom or
body cavity (12) and to the parietal lateral plate mesoderm or future ventral
body wall (9) that develops later. Other key landmarks are the hollow dorsal
nerve cord (5), notochord (1), and neural crest (4). Each somite is composed
of three zones around the myocoele cavity:
-
sclerotome at position 8,
-
dermatome at position 7, and
-
myotome at position 6.
In bovine embryos, the cells of the dermatome and myotome differ in their
arrangement so that the myocoele cavity may be merely an artifact produced
by the histological processing of embryos.
-
The sclerotome gives rise to the bone and cartilage of the vertebral column
and maintains its segmental arrangement.
-
Seen in a whole embryo, each somite appears as a cube of tissue, with left
and right somites forming pairs in an anterior to posterior sequence.
-
The division of continuous strips of mesoderm to become somites follows
a plan that is laid down much earlier in development by the way in which
cells are arranged.
-
The ectoderm eventually forms the epidermis of the skin while the underlying
connective tissue dermis is formed from the dermatome.
-
The sclerotome is composed of loosely packed and morphologically undifferentiated
mesenchyme cells. The mesenchyme surrounds the notochord and neural tube
and later differentiates to form cartilagenous precursors of the vertebrae.
-
In farm animals, myotomes become elongated from anterior to posterior,
and this obscures the original cuboidal shape of each myotome. Extensive
changes in the shape and orientation of somitic cells occur during the
development of the somites.
-
Premyoblasts give rise to myoblasts that fuse to form muscle fiber precursors
called myotubes. Myotubes have a "tube" of myofibrils around the axially
located nuclei, as seen in the three largest fibers of this cross section.
-
The axial muscles of the body, including the tongue and extraocular muscles,
are derived from the somitic mesoderm of the myotomes.
-
In many animals, the limb buds of the embryo become filled with mesenchyme
cells that have broken away from the parietal layer of the lateral plate
mesoderm. Thus, the origin of limb muscles is from the differentation in
situ of mesenchyme derived from lateral plate mesoderm. In the chick wing
and leg, however, myogenic stem cells may be derived from the somitic myotome.
-
The migration of somitic cells to a site of myogenesis in a limb is regulated
by the conditions within the developing limb bud and transplanted cells
follow the host pattern of muscle development. However, if wing buds are
experimentally isolated before being invaded by somitic cells, their own
mesoderm may differentiate into muscle. Thus, under some conditions,
the versatility of somatopleural cells enables them to develop into skeletal
muscle as well as connective tissue in the limb. In other words, here
we have the primary divergence of muscle tissue to become meat, from adipose
and connective tissue which modify the value of the meat in terms of connective
tissue toughness and marbling.
-
In experimental situations with cultured cells, non-myoblastic cells can
be induced to form functional myotubes by treating them with azacytidine.
-
The survival of somitic muscle in chick embryos is dependent on contact
with either neural tube or notochord, whereas limb muscles may survive
for longer . The optimum growth and differentiation of limb buds also depends
on a cellular contribution from adjacent somites. At least two precursor
stem cells contribute to each somite.
LIMB BUDS
Limb bud formation is regulated by a dialogue between ectoderm and mesoderm:
-
(1) the ectoderm makes an apical ridge in response to a message from the
mesoderm,
-
(2) the mesoderm grows to form parts of the limb in response to a message
from the ectoderm, and
-
(3) the mesoderm instructs the ectoderm to remain thick and to keep inducing
mesodermal growth.
The initial amount of premuscular mesoderm may be controlled by ectodermal
factors.
The mesoderm of the limb bud separates into muscle-forming and cartilage-forming
regions. The chondrogenic regions that give rise to cartilage
can be detected because they synthesize extracellular proteoglycan. Molecular
differentiation is preceded by differential vascularization so that vascular
growth also might be involved in establishing metabolic gradients in the
developing limb.
Very little is known about the factors that regulate the quantitative
distribution of premuscle mesoderm, and nothing is known about the role
of such factors in the regulation of the postnatal potential for muscle
growth in meat animals. For example, the maximum postnatal number of muscle
fibers might be predetermined by the number of stem cells and by the number
of times that their offspring divide. At present it appears likely that
the numbers of mitotic divisions in a cell lineage might be genetically
programmed, but with environmental factors controlling the rate of division.
Cell lines can also be traced back to the early development of the somites.
In poultry, for example, each somite makes specific contributions to the
development of particular muscles. The point at which multipotential
precursor cells differentiate to become myogenic stem cells is genetically
regulated by a single gene called myd. In other words,
is myd a primary control point for genetically-regulated muscle mass in
meat animals?
The cleavage of premuscle mesoderm in the limb buds of embryonic chicks
has been examined to establish muscle homologies as a basis for comparative
anatomy. The evolutionary origin of tetrapod limb muscles from the dorsal
and ventral muscle masses of ancestral fish fins is reflected in a primary
cleavage of premuscle limb bud mesoderm into dorsal and ventral masses.
Dorsal and ventral masses undergo a further sequence of cleavages (2 in
the lizard and 5 in mice) to form the individual muscles found postnatally.
These events are completed quite early in development; by 13.5 days in
mice, by 8 days in chicks, and by 17 mm crown-rump length (approximately
27 days gestation) in pigs. Cleavages to form future muscles may depend
on the presence of the skeleton, although osteofascial compartments such
as that of the avian supracoracoideus muscle may form in the absence of
any muscle tissue.
MITOSIS
The mesodermal cells of somites and limb buds undergo frequent mitosis,
with a variety of factors such as IGF-I and PDGF being mitogenic.
The peak of mitotic activity in the limb buds of the chick embryo is at
about 5 days incubation. Dividing cells are rounded in shape and are locked
into a mitotic cycle.
-
G1, (gap one) or rest after the last mitosis (2.0 hr)
-
S, synthesis of new DNA (4.3 hr)
-
G2, (gap two) or rest after DNA synthesis (2.4 hr)
-
M, mitosis (0.8 hr)
-
return to G1 or become a myoblast (5-7 hr)
The escape from this cycle, when a stem cell becomes a postmitotic myoblast,
appears to be irreversible. The cycle preceding a cell's escape has been
termed the quantal division. The number of times that a
clone of cells remains locked into the mitotic cycle might have a profound
importance on myoblast numbers: just one extra cycle by all cells might
double the number of myoblasts and give rise to extra muscle fibers (hyperplasia).
The population of premyoblasts capable of mitosis may not be completely
homogeneous since it might contain true stem cells and committed precursors.
A committed precursor is a cell that may give rise to a cohort of 16 terminally
differentiated muscle cells. Obviously, factors that may regulate myoblast
proliferation, such as triiodothyronine are extremely important
to the meat industry.
Another way of looking at this system of cell proliferation is to consider
cells at the escape point in their mitotic cycle. Both the daughter cells
produced by mitosis may stay in the cycle, both may escape to become myoblasts,
or one may stay in and one may escape. With a population of cells, the
percentage of escaping cells starts at 0% in very young embryos, before
the appearance of any myoblasts, and then increases towards, but never
reaches 100% (some stem cells remain as satellite cells, a source
of muscle nuclei during growth and regeneration). Cell populations containing
mixtures of premyoblast stem cells, mononucleate myoblasts and fused myoblasts
can be sorted with arabinocytidine. This prevents the formation
of new myoblasts but does allow cell fusion. In cultures from 11-day chick
embryos, about 20% of cells are myoblasts, but the percentage is lower
in younger embryos. Another way of sorting cells is to determine what percentage
may be cloned to give rise to myoblasts capable of fusion. Chick leg bud
mesoderm at 72 hours incubation contains 0%, at 80 hours it contains 10%,
and at 6 days it reaches 60%. In human limb buds, comparable values are
14% at 36 days, with a 90% plateau from 100 to 172 days.
Another factor controlling cell proliferation might be the duration
of the mitotic cycle, possibly by a variation of the duration of G1
. Cells that have escaped from the mitotic cycle to become myoblasts eventually
fuse together, but the fusion of cells eventually becomes less frequent,
as if inhibited. Alternatively, escape from the mitotic cycles may be in
late G1. Cells in G1 may respond to PROSTAGLANDIN E1
with a transient increase in intracellular cyclic AMP. This may activate
protein kinase and the onset of myoblast fusion. As discussed later, the
nervous system exerts some regulation over muscle development, and its
control over myoblast proliferation is probably achieved by varying the
duration of G1 rather than G2. Because of the importance of
G1 in the regulation of cell numbers, it is interesting to note that the
G1 -S boundary is the point at which the cell synthesizes calmodulin. Calmodulin
is a protein that binds calcium ions, and which is thought to be involved
together with cyclic AMP in the regulation of many aspects of cell metabolism,
growth and division.
MYOBLASTS

The morphological features of postmitotic cells and of myoblasts prior
to fusion are not unlike those of other types of precursor cells in the
embryo. RNA synthesis dominates cell activity and results in a large ovoid
nucleus, prominent nucleoli (which vary in number between species), diffuse
chromatin and many ribosomes. Myoblasts are bipolar spindle-shaped cells,
whereas fibroblasts tend to be triangular in shape. Myoblasts may form
tight junctions where they are in contact with each other, usually at the
tips of their elongated cytoplasmic extensions. Myoblasts may be categorized
into three types or cellular isoforms.
-
Type I myoblasts are the earliest and fuse to form small myotubes with
four to six nuclei.
-
Finally, Type III myoblasts are formed only after the muscle becomes innervated.
In the chick embryo, there are three different cell lineages of myoblasts
early in myogenesis (with fast-contracting myosin, with mixed fast and
slow, and with slow) that are independent of any innervation. The non-neural
cues that initiate myogenesis appear to originate externally to the future
muscle and are, in some way, related to position within the embryo. Later
in development there are myoblasts whose developmental fate is determined
by the nervous system.
The overall sequence of events in myogenesis may be separated into commitment,
differentiation and maturation.
-
Commitment occurs when a pluripotential mesodermal cell has its future
restricted to myogenesis,
-
differentiation is marked by the transcription of genes coding for typical
features of the skeletal muscle fiber, and
-
maturation or terminal differentiation occurs after innervation.
Myogenin and MyoD are genes in a family that is activated at commitment
to a myogenic lineage and could be very useful in exploring the factors
that determine muscle size in meat animals. Myogenin and MyoD are sensitive
to thyroid hormones, as well as being regulated by muscle electrical activity,
possibly via a mechanism dependent on cyclic-AMP. Innervation controls
the abundance of myogenic factors such as MyoD1 and myogenin, and denervated
muscle reverts to a neonatal phenotype. Subject to neural regulation, MyoD
is prevalent in fast muscles, and myogenin in slow muscles.
As attractive as direct genetic regulation of muscle fiber numbers may
be to meat scientists, it is important not to overlook other possibilities.
Transforming growth factor beta 1 (TGF-þ1) is a small peptide involved
the joint develop of muscle fibers and connective tissues. Following local
induction of TGF-þ1, it may produce local gradients that enhance
the development of connective tissues by fibroblasts, but inhibit myogenesis.
Thus, a reduction of TGF-þ1 gradients might produce a condition similar
to that found in double-muscled cattle.
Myoblast fusion
Myoblasts fuse with each other to form multinucleate cells that give rise
to multinucleate skeletal muscle fibers. Fusion is initiated at a single
site between two myoblasts. The pore formed to link adjacent cells enlarges
and leaves no trace of the intervening membranes. The cytoskeleton within
myoblasts forms a dense meshwork under the cell membrane and undergoes
extensive remodelling at the time that myoblasts fuse to form myotubes.
Myofibrils are formed rapidly once fusion has occurred and they accumulate
under the cell membrane. The nuclei become restricted to an axial core
of sarcoplasm surrounded by myofibrils arranged to form a hollow cylinder
or tube. At this stage, the whole cellular structure may be called a myotube
because its structure is dominated by the hollow cylinder of myofibrils.
Myoblasts do not normally fuse with other types of cells but, experimentally,
myoblasts of one species can be induced to fuse with myoblasts of another
species.
Myoblast fusion has been observed by time-lapse cinematography, where
some myoblasts may be seen to move to suitable positions prior to fusion
while, in other cases, this may be unnecessary because aggregates of dividing
cells have kept in contact with fused cells. Fusion is preceeded by a period
of cell to cell recognition in which the cells may still be dispersed chemically
with EDTA. Recognition is followed by a period of adhesion in which trypsin
must be added experimentally in order to disperse the cells. Finally, after
membrane fusion, fused cells cannot be dispersed. Cultured myoblasts fuse
when their numbers reach a certain density, perhaps in response to a chemical
signal. Within the myoblast, an increase in the level of cyclic AMP
initiates the events that lead to fusion. Myoblasts have surface antigens
that are probably involved in cell-cell recognition. Myoblast fusion is
triggered by calcium ions but is inhibited by magnesium and potassium ions.
Formation of myofibrils
The synthesis of all the major proteins of the myofibril is simultaneous
but a number of different sized filaments that may occur in myogenic cells.
The myosin and actin of developing myofibrils appear as 15 to 16
nm diameter and 5 to 6 nm diameter filaments, respectively. The diameter
of unincorporated myosin filaments is similar to those that have already
joined a myofibril. The filaments of the Z line are 5 to 6 nm in diameter.
Filaments with diameters of 5 to 6 nm also occur below the cell membrane,
but are probably non-muscle actin. Microtubules, with diameters from 22
to 25 nm, may be found in the axial core of myotubes. Microtubule subunits
with a diameter of 10 microns may also be found. Filaments with diameters
from 5 to 10 nm may be found at the myotendon junction.
There are numerous possibilities for the method of assembly of myofibrils,
and I keep an open mind on which is the most likely.
-
The formation of T tubules might begin before their connection with the
plasma membrane is established, but the origin of T tubules is still not
universally agreed. The two basic ideas, (1) that the tubule invaginates
from the membrane surface, or (2) that it may be formed internal to the
membrane by the fusion of vesicles, are not incompatible and may be complementary.
Sarcoplasmic reticulum establishes its relationship with Z-lines early
in development then, as T tubules develop inwards from the plasma membrane,
triads are formed that later associate with the A-I junction.
Many of the earlier studies on the early formation of sarcomeres must be
reconsidered to take into account the respective contributions of alpha
actinin, desmin and vimentin. At present, it appears that
the regular structure and arrangement of sarcomeres proceeds in two stages.
The initial formation of Z lines containing alpha actinin is dependent
on a process of trial and error to establish alignment and is followed
by the appearance of desmin and vimentin at the time that the lateral alignment
of Z lines is established.
-
Vinculin is involved in holding thin filaments onto the cell membrane
before sarcomere arrangement is apparent.
-
Filamin, a protein normally found in smooth muscle, may also make
a transient appearance when Z lines are being assembled.
-
The initial aggregation of thick and thin filaments also may be organized
by stress fiber-like structures (SFLS). SFLS are thought to be composed
of microfilaments together with contractile proteins and may provide a
transient sarcomeric scaffold against which each myofibril is assembled.
Thus, the early formation of intermediate filaments forms an anisotropic
matrix in which thick and thin filaments later appear.
-
Possibly there are two organizing centers in the assembly of sarcomeres:
one for the A band (thick filaments, C-protein and myomesin) and one for
the I-Z-I complex.
-
M-lines are formed early in development and later become structurally differentiated
in fast and slow muscle fibers.
DETERMINATION OF MUSCLE FIBER ARRANGEMENT
The first myotubes formed in each embryonic muscle are involved in establishing
the future arrangement of muscle fibers, as well as in establishing the
approximate size and anatomical location of the muscle. Little is known
about any of these three factors in meat animals.
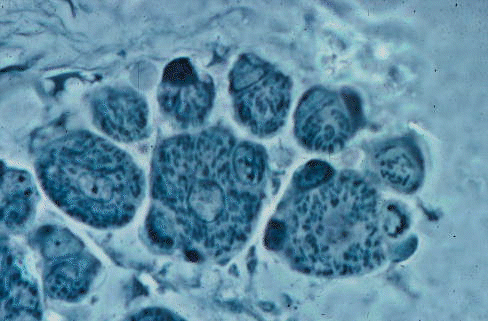
 Early myotubes
Early myotubes
The major nerve trunks grow into a limb bud by following the connective
tissue framework of the bud, but developing muscles may be necessary to
invoke the formation of side branches to the muscle. Muscle fibers themselves
may not be absolutely essential since the formation of nerves can be induced
by the general growth pattern of somatopleural derivatives in the absence
of muscle. Muscles with more than about ten myotubes might be able to initiate
the formation of a nerve branch to the muscle.
Muscles may be attached to either the shaft (diaphysis) or the knob
(epiphysis) of a bone. But the longitudinal growth of bones occurs at cartilagenous
epiphyseal plates, and one of these plates is located between each epiphysis
and its diaphysis. Thus, to retain their positions relative to each other
during epiphyseal plate growth, some muscle attachments must migrate over
the bone surface. Muscle migrations are regulated by the bone and traction
by the periosteum is responsible for the migration of tendon insertions.
Muscle development in the limbs of fetal pigs may be shaped by a dynamic
interaction between linear skeletal growth and the resistance of muscles
to stretching. The nervous system appears to have no direct part in the
determination of myotube alignment.
If muscle stretching really does shape muscle growth, the determination
of muscle fiber arrangment might be explained by the contact guidance theory
that attempts to explain how nerve cells invade developing tissues. Myotubes
and myoblasts might be guided by a matrix of very fine connective tissue
fibers. Migrating myogenic stem cells in chick embryos branch into filopodia
at their leading edges, and stem cells follow the alignment of fine connective
tissue fibers. The ends of myotubes actively grow through the tissue of
the future muscle and have a well developed cytoskeleton dominated by microtubules.
Molecules of fibronectin have binding sites for a number of the components
that surround cells (such as for collagen and glycosaminoglycans) but also
they can bind to the surfaces of cells. Thus, matrices of fibronectin may
be involved in the guiding of cell migrations and the determination of
muscle architecture. The initial arrangement of connective tissue fibers
probably is organized by fibroblasts that are able to use their intracellular
microfilaments to exert a force on the surrounding extracellular matrix.
It is likely that a number of other substances also are involved in the
determination of cellular arrangement in developing muscles, such as glycoprotein
complexes liberated by fibroblasts.
In vitro, myoblasts only develop a parallel alignment if they are cultured
on a type of collagen that forms distinct collagen fibers. Myotubes also
may be pulled into alignment by their already anchored ends to follow the
dominant directions of a stretched matrix, and the chances of myoblast
fusion are increased when myoblasts become aligned on parallel collagen
fibers.
The angular arrangement of fibers is more difficult to explain. Perhaps
the tensile forces that shape the connective tissue matrix of a pennate
muscle are transmitted by intramuscular tendons. Another possibility is
that myoblast arrangement is influenced by the orientation of electrical
fields. Cultured myoblasts become arranged with their long axes perpendicular
to electric fields of 36 to 170 mV/cm.
Intracellularly, the parallel arrangement of myofibrils is dependent
on the proper attachment of the whole cell. New aggregates of thick
and thin filaments appear first at the periphery of cells so that the longitudinal
orientation of filaments may follow the direction of membrane stretching.
Many of the early histologists who studied myogenesis were impressed
by the widespread evidence of cellular degeneration (retrograde
metamorphosis) that they found in developing muscles. Lysosomes capable
of causing degeneration are well developed even in myoblasts. More recently,
there has been a trend to dismiss degenerative phenomena during myogensis
as being a mere consequence of localized myotube contracture (a sustained
and destructive contraction). An alternative viewpoint is to regard contracture
followed by degeneration as a means of eliminating fibers that have failed
to align themselves correctly within a muscle. If cultured intercostal
muscles are maintained between pieces of rib, when the muscles are stretched
by slow separation of the ribs, muscle fibers continue to develop but,
when they are not stetched, the fibers degenerate. The passive stretching
of myotubes activates the sodium ion pump of their membranes, and this
is followed by increases in amino acid uptake and protein synthesis. In
vitro, muscle fibers maintained in a relaxed state by tetrodotoxin exhibit
a normal accumulation of vimentin and desmin, but do not accumulate contractile
myofibrillar proteins. The stimulation of amino acid transport and protein
synthesis induced by the stretching of myotubes acts through some mechanism
that is intrinsic to the myotube and which does not rely on circulating
hormonal factors. And mechanical stimulation may act on protein synthesis
and muscle growth by the release of second messengers such as arachidonic
acid, diacylglycerol and prostaglandins. Long-term cultures of muscle
that become connected to their substrate and are able to contract then
are able to develop structures that resemble myotendinous junctions, as
well as endo-, peri- and epimysial layers of connective tissue.
During the determination of fiber arrangement, a loss of cells by retrograde
metamorphosis may affect muscle size, as in the development of the extrinsic
ocular muscles that rotate the eyeball. Transplantation of developing eyes
from a large-eyed amphibian species to a small-eyed species results in
an enlargement of the host's extrinsic ocular muscles. The cause of muscle
enlargement is an increase in fiber numbers, hyperplasia.
MASS PRODUCTION OF MUSCLE FIBERS
In the descriptions of myogenesis given by many histology textbooks, we
may read that all skeletal muscle fibers of newborn animals are derived
from myotubes by the radial migration of nuclei and a disruption of the
tubular arrangement of myofibrils. In pigs, however, the great histologist
Theodore Schwann (way back in 1839!) discovered that two types of muscle
fiber precursors exist in the fetus: one type has a tubular arrangement
of its myofibrils and may be called a myotube, while the other type lacks
a tubular appearance and cannot reasonably be called a myotube. As yet,
there is no generally agreed name for this second type of muscle fiber
precursor. Here it is called a secondary fetal muscle fiber, shortened
to secondary fiber.
 Secondary fibers.
Secondary fibers.
The classical myotube may then be called a primary fiber or primary
myotube. Unfortunately, this is the reverse of Schwann's original terminology
since he worked backwards from late to early embryos. The duality of muscle
fiber precursors was almost completely ignored until recently, now there
are gangs of people rushing around who claim the discovery.
The myoblasts that contribute to the formation of secondary fibers
are derived from a special cell lineage distinguished by a developmental
dependency on their innervation.
There is no general agreement yet on the histological significance of
secondary fibers. The viewpoint taken here is that secondary fiber formation
is a process for the rapid mass production of relatively large numbers
of new muscle fibers, taking advantage of two factors; firstly, that the
general features of muscle architecture have already been established by
the arrangement of primary myotubes and, secondly, that the surfaces of
gently contracting myotubes provide an ideal site for myoblast contact
and fusion. Myoblasts are commonly found clinging to myotubes. As myotubes
contract, these surface myoblasts probably bump against each other. The
dimensional changes that occur on the myotube surface, as it decreases
in length and increases in radius, favor myoblast contact along the length
of the myotube. Thus, the long axis of fused myoblasts will follow that
of their supporting myotube. With an in vitro model created by stretching
the substrate of cultured avian myoblasts, the optimal rate of stretching
for maximum myoblast alignment is 0.2 mm/hour.
Strings of fused myoblasts that have been assembled on a primary myotube
are soon reinforced continuously along their length by new myofibrils.
New myofibrils are grouped as a solid core, mostly located away from the
supporting myotube. Most secondary fibers formed in this way retain a more
or less axial core of myofibrils rather than having a tubular arrangement
of their myofibrils. Secondary fibers adhere to their primary myotubes
by means of pseudopodial processes that project into invaginations on the
surface of the primary myotube. Further contractions of the supporting
myotube (as indicated by their short sarcomeres), do not spread to secondary
fibers (as indicated by their long sarcomeres). Once a secondary fiber
has acquired a substantial core of fibrils, differences in length due to
contraction may create a shear force between the secondary fiber and its
supporting myotube that leads to the separation of secondary fibers from
their supporting myotubes, and accounts for the fact that secondary fibers
are often sinuously folded when their myotubes are contracted. 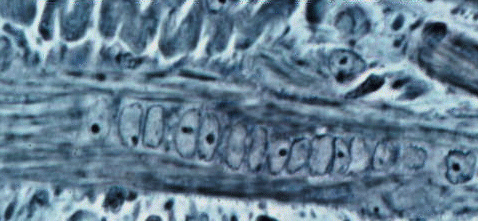 Sinuous
secondary splitting off its primary.
Sinuous
secondary splitting off its primary.

The morphological categorization of prenatal muscle fibers into primary
myotubes and secondary fibers is a general principle which, at best, can
only account for a majority of cases. At worst, it does not take long to
find a few fibers with features that are intermediate between those of
primary and secondary fetal fibers. Schwann in 1839 was the first to notice
these transitional cases. Why should early fibers have a tubular structure
and later fibers be different? Early in muscle development, strings of
myoblasts start to produce filaments below their plasma membrane. If sustained
by tension from successful terminal attachments, the continuous peripheral
formation of new filaments gives rise to a complete tube of myofibrils
below the plasma membrane. This radial symmetry provides the maximum surface
area for mass production of secondary fibers. As secondary fibers start
to acquire fibrils that do not contract synchronously with those of the
supporting myotube, shear forces may develop between supporting myotubes
and secondary fibers and further proliferation of fibrils in the secondary
fibers may lead to their separation.
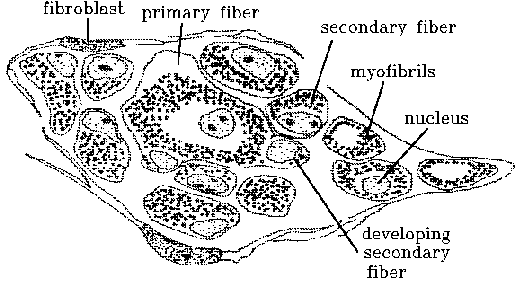
Now we can understand the smaller fibers in this image we saw before. 
Once a secondary fiber has separated, its core of fibrils may become
crescent-shaped in transverse section as the secondary fiber starts to
utilize the free space below the membrane originally apposed to the supporting
myotube. The oldest secondary fibers, which are now pushed away from their
parent myotubes by younger secondary fibers, start to assume a tubular
structure. Perhaps now they may support production of further secondary
fibers themselves, but secondary fiber production slows down as fetal development
nears completion. The axial nuclei of myotubes move, or are pushed to a
peripheral position below the cell membrane, and the morphological distinction
between primary myotubes and secondary fibers is obscured.
The radial dimensions of primary and secondary fibers are difficult
to measure histologically. Primary myotubes may appear to become smaller
during postnatal development, while secondary fibers may remain constant
in size until just before birth. However, the decrease in mean size of
primary myotubes may be related to their less frequent contraction or to
the detachment of secondary fibers. Similarly, the mean size of secondary
fibers may be biased by the constant addition of newly formed fibers with
a small diameter. However, just before before, the radial growth is secondary
fibers is unmistakable.
It is difficult to estimate the numbers of secondary fetal fibers formed
by mass production. The fibers that can be counted in a muscle cross section
(apparent number) comprise only a fraction of those present in the whole
muscle (real number) so that the apparent numbers of primary myotubes and
secondary fibers only indicate the ratio of primary myotubes to secondary
fibers. Even the interpretation of this ratio rests on the unproved assumption
that both types of fibers maintain equal or constantly proportional lengths.
Even the apparent ratio of primary myotubes to secondary fibers is difficult
to determine towards the end of prenatal development because a few of the
oldest secondary fibers may develop a tubular structure at the same time
that primary myotubes start to lose their tubular structure.
The ratio of tubular to nontubular fibers describes a curve that starts
with all tubular fibers and ends with all nontubular fibers. From this
curve, the start of secondary fiber production may be estimated at approximately
55
days gestation in pigs. The end of secondary fiber production is more
difficult to pinpoint: subjective estimates range from 70 days, through
85 to 95 days, to 100 days, depending on whether secondary fibers are counted
while they are still on their supporting myotubes or when they are first
detached. These estimates are based on studies using three different methods
of embedding (thin epon sections, frozen sections and paraffin sections,
respectively) - another factor that may have influenced the estimates.
One way to interpret the curve of the ratio of tubular to nontubular
fibers is to regard the rapid change in ratio from 55 to 70 days as a consequence
of secondary fiber mass production and the slow change in ratio from 70
to 110 days as a consequence of nuclear migration. Thus, at 70 days in
porcine muscle, it appears that each primary myotube has supported the
production of approximately five secondary fibers.
Differences between genetically obese and genetically lean pigs become
apparent at 80 days gestation for muscle DNA in the semitendinosus and
at 90 days onwards for muscle weight. In other words, differences are detected
soon after the mass production of secondary fibers is complete. Secondary
fiber formation is reduced in runt piglets. In rats, where the effects
of a restricted maternal nutrition during gestation and lactation are more
easily induced than in farm animals, restriction causes a reduction in
the numbers of secondary fibers but not of primary myotubes.
In fetal calves, secondary fiber formation is completed by 20 cm crown-rump
length, at about 205 days gestation. In chick embryos, primary myotubes
are all formed by approximately 11 days and the mass production of secondary
fibers occurs up to 16 days of incubation.
primary myotubes ---> red fibers
secondary myotubes ---> white fibers
although there is plenty of plasticity so muscles can adapt functionally.
 This diagram shows a greatly
simplified plan of a transverse section through an embryo. The bottom of
the figure, in particular, bears little or no resemblence to either mammalian
or avian embryos but is included to indicate the general relationships
of the somites to the endoderm of the future gut (11), to the coelom or
body cavity (12) and to the parietal lateral plate mesoderm or future ventral
body wall (9) that develops later. Other key landmarks are the hollow dorsal
nerve cord (5), notochord (1), and neural crest (4). Each somite is composed
of three zones around the myocoele cavity:
This diagram shows a greatly
simplified plan of a transverse section through an embryo. The bottom of
the figure, in particular, bears little or no resemblence to either mammalian
or avian embryos but is included to indicate the general relationships
of the somites to the endoderm of the future gut (11), to the coelom or
body cavity (12) and to the parietal lateral plate mesoderm or future ventral
body wall (9) that develops later. Other key landmarks are the hollow dorsal
nerve cord (5), notochord (1), and neural crest (4). Each somite is composed
of three zones around the myocoele cavity:
 Premyoblasts
Premyoblasts


 Early myotubes
Early myotubes
 Secondary fibers.
Secondary fibers.
 Sinuous
secondary splitting off its primary.
Sinuous
secondary splitting off its primary.

