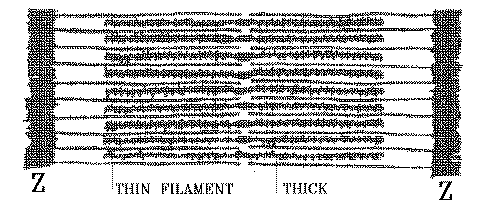

THE THICK FILAMENT IS MADE OF MYOSIN MOLECULES ARRANGED LIKE THIS -

So this is the unpacked arrangement shown above. Close them together laterally, and they would form a long bundle - the thick filament.

The sarcoplasmic reticulum surrounds the fibrils within a fibre and occupies about 4% of the volume of the muscle fibre. In a resting muscle fibre in a live animal, the cytosol is kept almost free of calcium ions by the rapid capturing or sequestering of calcium ions by the sarcoplasmic reticulum. The concentration of calcium ions in the cytosol of a resting muscle is about 5 x 10-8 M, which increases to about 5 x 10-6 M when the muscle contracts. The protein calsequestrin binds and stores calcium ions within the sarcoplasmic reticulum, and the calsequestrin concentration is higher in fast-contracting muscle fibres than in slow fibres.
(1) Voluntary activity from the brain or reflex activity from the spinal
cord computes that a contraction is needed.
(2) The impulse is passed down the spinal cord to a motor neuron, and an action potential passes outwards in a spinal nerve, carried by an axon linking the motor neuron to all its muscle fibers.
(3) The axon branches to supply all its muscle fibres (motor unit),
and the action potential is conveyed to a neuromuscular junction on each
muscle fibre.
(4) At the neuromuscular junction, the action potential causes the release
of packets or quanta of acetylcholine into the small space (synapse) between
the axon and the muscle fibre.
(5) Acetylcholine causes the electrical resting potential of the muscle fibre membrane to change, and this then initiates a new action potential that passes in both directions along the surface of the muscle fibre.
(6) The action potential spreads deep inside the muscle fibre, carried
by transverse tubules.
(7) Where transverse tubules touch parts of the sarcoplasmic reticulum,
the sarcoplasmic reticulum releases calcium ions.
(8) The calcium ions cause the movement of troponin and tropomyosin on their thin filaments, which then enables the myosin molecule heads to "grab and swivel" their way along the thin filament.
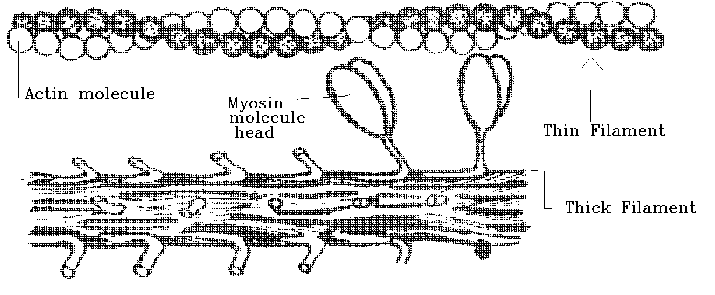
(9) The acetylcholine at the neuromuscular junction is destroyed by
an enzyme (acetylcholinesterase), and this terminates the stream of action
potentials along the muscle fibre surface.
(10) The sarcoplasmic reticulum ceases to release calcium ions, and
immediately starts to resequester all the calcium ions that were just released.
(11) Without calcium ions, a change in the configuration of troponin
and tropomyosin blocks the action of the myosin molecule heads so that
they cannot reach the thin filaments any more, and contraction ceases.
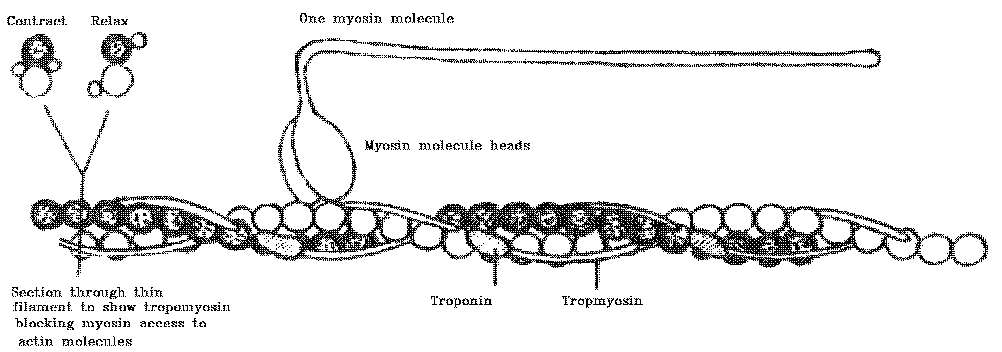
(12) In the living animal, an external stretching force, such as gravity or an antagonistic muscle, is required to pull the muscle back to its original length.
(1) As rigor develops after slaughter, carcass muscles may be stretched
or contracted, depending largely on their position in the hanging carcass.
(2) Relaxed muscles produce meat that is more tender than that from
contracted muscles.
(3) Rapid cooling before the start of rigor causes muscles to shorten
(cold shortening). Sequestering calcium ions takes a lot of energy, so
when the sarcoplasmic reticulum is cooled down, its efficiency drops, and
it cannot then mop up all the calcium ions released by reflex muscle activity
during slaughter and by leakage through the sarcoplasmic reticulum membrane.
(4) Freezing of meat before the completion of rigor causes extreme shortening
when meat is thawed (thaw shortening), because ice crystals have slashed
open the sarcoplasmic reticulum allowing massive contraction once the system
is warm enough to respond.
To avoid cold shortening, meat must not reach 10oC within 10 hours post mortem in lamb carcasses. Beef sides should not be exposed to air below 5oC or faster than 1 metre / second within 24 hours after slaughter. Although thaw shortening causes extreme toughness in beef, the situation is different in poultry. Poultry meat toughness reaches its maximum level as recently slaughtered carcasses are washed, but the meat becomes more tender as carcasses are chilled. Maximum tenderness is reached after blast-freezing and thawing.
Meat tenderness and taste definitely are improved if carcasses or vacuum
packed cuts are conditioned after slaughter but, paradoxically, beef is
quite tender just two hours after slaughter, and several days of conditioning
are required to recover this degree of tenderness. After this point, the
beef becomes progressively better, with a higher temperature allowing a
faster rate of conditioning. Gains in tenderness from conditioning are
particularly important in grass-fed beef. When beef is from youthful animals
without risk of being cold shortened, then lack of conditioning is a serious
cause of beef toughness.
In early research, it was though that increased tenderness might originate
from the breakdown of rigor bonds between thick and thin filaments, but
now this appears unlikely, because increased tenderness during conditioning
still occurs when sarcomeres are at a stretched length that virtually eliminates
any overlap of thick and thin filaments. Another factor that contributes
to the increased tenderness of conditioned meat may be an increase in ionic
strength that solubilizes myofibrillar proteins, particularly those of
the thick filament.
Calcium-activated protease (Calpain) is an enzyme located in the cytosol.
It slowly disrupts Z lines by releasing alpha actinin, a protein that holds
the thin filaments into the Z line. This makes an important contribution
to meat conditioning, but many aspects of the calpain system still are
unknown. Calpains occur in all vertebrate cells, where they are involved
in general purpose enzymatic activity related to maintenance of cell shape
and the response of cells to hormones. Also there are other enzymes inside
the muscle fibre which might be involved in the conditioning effect. Cathepsin
B and D occur in lysosomes (suicide bags to destroy unhappy cells) and
parts of the sarcoplasmic reticulum. Acid phosphatase is another enzyme
that has been found inside muscle fibres.
The enhancement of meat taste and aroma during conditioning still is not fully understood. Conditioning causes changes in a number of water-soluble compounds that affect meat taste, including free amino acids, metabolites of ATP, organic acids and sugars. Free amino acids released by aminopeptidase activity are increased during the conditioning of pork, chicken and beef, although the changes in beef are less than anticipated.
Some of us are worried that conditioning might be reduced by factors
in modern animal production, such as the genetic selection for rapid growth
rate. Even animals that are growing very rapidly have a balance between
the synthesis of new tissue (anabolism) and the break-down of worn or damaged
tissue components (catabolism). Thus, a young animal with rapid growth
has much more anabolism than catabolism. But when it reaches its full adult
size, anabolism and catabolism are equal. If selection for rapid growth
has been achieved by decreasing catabolism as well as increasing anabolism,
then we may find that we have weakened the system responsible for the favourable
effects of conditioning. Perhaps we are worrying about nothing and there
is no problem, but that is what the cod fishermen said about over-fishing,
right up until the time that the cod disappeared.