FIBROUS CONNECTIVE TISSUE
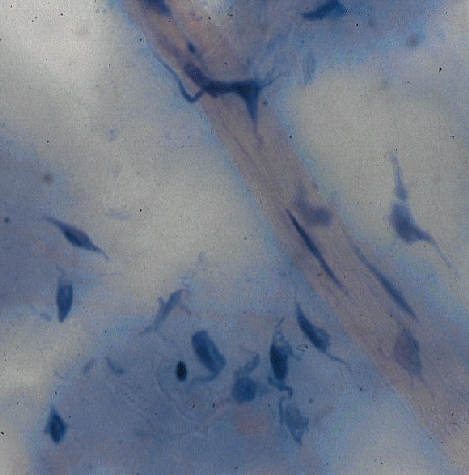
Three essential features: (1) cell, (2) fibres, and (3) matrix.
Introduction
Dictionaries do not always give the right definitions of things, especially
in relation to the meat trade, and it would be foolish to think that sweetbreads
originate from pancreas rather than thymus just because it says so in some
dictionaries (presumably compiled by vegetarians). Even though most dictionaries
dictate that gristle is the same thing as cartilage, I disagree. Apart
from the odd bit of scapular, joint, or costal cartilage, there is virtually
no cartilage in most meat cuts, yet all my life I have found chewy strands
of connective tissue in tough cooked meat and I have called them "gristle".
Whatever name you may give them, I am sure you know what I am writing about,
and will agree that we need to understand the scientific basis of the fibrous
connective tissues in meat.
 The fibrous connective
tissues in meat form a continuous mesh, as shown in the image to the left,
from the microscopic strands of endomysium around individual muscle fibers,
to the larger layers of perimysium that delineate bundles of muscle fibers,
all being gathered and connected to the thick, strong epimysium on the
surfaces of individual muscles.
The fibrous connective
tissues in meat form a continuous mesh, as shown in the image to the left,
from the microscopic strands of endomysium around individual muscle fibers,
to the larger layers of perimysium that delineate bundles of muscle fibers,
all being gathered and connected to the thick, strong epimysium on the
surfaces of individual muscles.
The image below shows a thick layer of perimysium.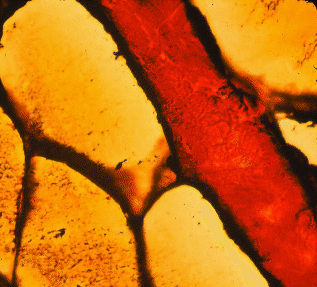
The endomysium, perimysium and epimysium contain two types of
protein fibers, collagen and elastin, which now we will consider in detail.
Collagen fibers
Collagen is an elongated protein that forms extremely strong but very small
fibrils
(best seen with an electron microscope). Lots of these collagen fibrils
are bound together to form collagen fibers that easily can be seen
with a light microscope. When collagen fibers form sheets or cables we
can see them in the meat without a microscope, and we may detect them as
gristle if they do not gelatinize in cooked meat.
Collagen is the most abundant protein in the animal body, and the collagen
which occurs in meat may be an important source of meat toughness. Beef
carcasses have to be graded by age mainly because of age-related changes
in collagen that cause meat from older cattle to be tough.
Large amounts of collagen are found in animal skin. In pig skin, for
example, collagen fibers are tightly woven from two directions to form
a tightly woven meshwork. Collagen is a raw material for major industries
in leather, glue and cosmetics.
Under a light microscope, collagen fibers in the connective tissue framework
of meat range in diameter from 1 to 12 micrometres (0.001 millimetre =
1 micrometre). They do not often branch and, when branches are found, they
usually diverge at an acute angle. Collagen fibers from fresh meat are
white, but usually they are stained in histological sections for examination
under a microscope. The most common stain for light microscopy is eosin,
which stains collagen fibers pink. Unstained collagen fibers may be seen
by polarized light since they are birefringent (light that comes through
Polaroid sunglasses is polarized, with all its waves in one plane; birefringent
means having two refractive indices). By rotating the plane of polarized
light, collagen fibers appear bright against an otherwise dark background
(when two Polaroid lenses are perpendicular they block most of the light,
but collagen fibers can rotate the light so that they appear bright). The
birefringence of collagen fibers in meat is lost at the point during heating
when gelatinization occurs. Collagen fibers have a wavy or crimped appearance
which disappears when they are placed under tension.
Collagen fibers fluoresce with a blue-white light when excited
with UV light so that the amount of connective tissue on a cut meat surface
may be measured very rapidly. Peak excitation is around 370 nm so that
the prominent 365 nm peak emission of a mercury arc lamp may be used. Some
indication of collagen fiber diameter may be obtained by spectrofluorometry
(measuring the wavelengths of fluorescence) because the fluorescence is
quenched (fades) fairly rapidly. Thus, large collagen fibers retain a central
core with a pre-quenching emission spectrum for longer than small fibers.
Fat only fluoresces weakly, to about the same extent as areas of muscle
with a low connective tissue content. This is how the connective tissue
content of meat may be measured with a fiber-optic probe for the on-line
detection of tough beef. Collagen fluorescence increases with animal age
so that probe measurements may have a promising
future in beef grading, and fiber-optic probe measurements now have been
correlated with consumer taste panel evaluation of chewiness in beef .
It is important remember, however, that a probe
for connective tissue cannot account for toughness caused by short sarcomeres
or inadequate aging of the meat!
Electron microscopy reveals that collagen fibers are composed of parallel
bundles of small fibrils with diameters ranging from 20 to 100 nm (0.001
micrometre = 1 nanometre). Collagen fibrils typically have diameters which
are multiples of 8 nm that may show the manner in which they grow radially.
Collagen microfibrils (even smaller structures that make up fibrils) may
appear to have a tubular structure with an electron-lucent lumen (appearing
empty under the electron microscope).
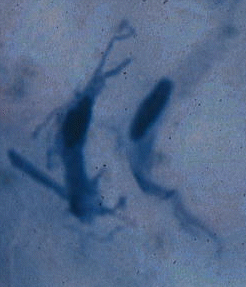 Collagen fibrils
are formed from long tropocollagen molecules which are staggered in arrangement
but tightly bound laterally by covalent chemical bonds. For electron microscopy,
when negatively stained with heavy metals that spread into the spaces between
the ends of molecules, collagen fibrils appear to be transversely striated.
The periodicity of these striations is 67 nm but often shrinks to 64 nm
as samples are processed for examination. Although collagen fibers are
located outside the cell, the initial stages of collagen fibril
assembly may be within the cell, with fibril morphology being regulated
by a special site on the fibroblast membrane (cells that form connective
tissue fibers are called fibroblasts).
Collagen fibrils
are formed from long tropocollagen molecules which are staggered in arrangement
but tightly bound laterally by covalent chemical bonds. For electron microscopy,
when negatively stained with heavy metals that spread into the spaces between
the ends of molecules, collagen fibrils appear to be transversely striated.
The periodicity of these striations is 67 nm but often shrinks to 64 nm
as samples are processed for examination. Although collagen fibers are
located outside the cell, the initial stages of collagen fibril
assembly may be within the cell, with fibril morphology being regulated
by a special site on the fibroblast membrane (cells that form connective
tissue fibers are called fibroblasts).
Tropocollagen molecules
Tropocollagen is a high molecular weight protein (300,000 Daltons) formed
from three polypeptide strands twisted into a triple helix. Each strand
is a left-handed helix twisted on itself, but the three strands are twisted
into a larger right-handed triple helix. The triple helix is responsible
for the stability of the molecule and for the property of self-assembly
of molecules into microfibrils. The flexible parts of each strand projecting
beyond the triple helix (telopeptides) are responsible for the bonding
between adjacent molecules. In other words, the cross links that bind
tropocollagen molecules together laterally are made between the helical
shaft of one molecule and the non-helical extension of an adjacent molecule.
In the polypeptide strands, the small amino acid glycine occurs
at every third position, and proline and hydroxyproline account for 23%
of the total residues. The regular distribution of glycine is required
for the packing of tropocollagen molecules and has been claimed as evidence
that all animals are derived by evolution from a single ancestral stock,
since the chance development of this unique regularity in unrelated animals
is thought unlikely. Hydroxyproline is quite rare in other proteins of
the body, and an assay for this imino acid (an imino acid is chemically
similar, but not the same as an amino acid) provides a measure of the collagen
or connective tissue content in a meat sample. Tropocollagen also contains
a fairly high proportion of glutamic acid and alanine as well as some hydroxylysine.
Biochemical types of collagen
Each tropocollagen molecule is composed of three alpha chains but 19 unique
alpha chains have been identified, giving rise to 11 different types of
collagen. These may be categorized into three general classes:
(1) molecules with a long (about 300 nm) uninterrupted helical domain,
(2) molecules with a long (300 nm or greater) interrupted helical domain,
(3) short molecules with either a continuous or an interrupted helical
domain.
The various types of collagen of interest in understanding the structure
of meat are as follows.
-
Type I collagen forms striated fibers between 80 and 160 nm in diameter
in blood vessel walls, tendon, bone, skin and meat. It may be synthesized
by fibroblasts, smooth muscle cells (around blood vessels) and osteoblasts
(bone-forming cells).
-
Type II collagen fibers are less than 80 nm in diameter and occur in hyaline
cartilage and in intervertebral discs. It is synthesized by chondrocytes
(cartilage-forming cells).
-
Type III collagen forms reticular fibers in tissues with some degree of
elasticity, such as spleen, aorta and muscle. It is synthesized by fibroblasts
and smooth muscle cells, contributes substantially to the endomysial connective
tissues around individual muscle fibers, provides a small fraction of the
collagen found in skin and occurs in the large collagen fibers dominated
by Type I collagen. It may have some function in regulating collagen fiber
growth.
-
Type IV collagen occurs in the basement membranes around many types of
cells and may be produced by the cells themselves, rather than by fibroblasts.
Although basement membranes were once regarded as amorphous (like glue),
many of them now are thought to be composed of a network of irregular cords.
The cords contain an axial filament of Type IV collagen, ribbons of heparin
sulphate proteoglycan, and fluffy material (laminin, entactin and fibronectin).
Type IV collagen occurs in the endomysium around individual muscle fibers.
Instead of being arranged in a staggered array, the molecules are linked
at their ends to form a loose diagonal lattice.
-
Type V collagen is found prenatally in basement membranes and cultures
of embryonic cells. It is synthesized by myoblasts (muscle-forming cells),
smooth muscle cells and, possibly, by fibroblasts. Type V collagen has
also been found in the basement membranes of muscle fibers, except at the
point where muscle fibers are innervated.
-
Type VI collagen is a tetramer of Type VI. It forms a filamentous network
and has been identified in muscle and skin. The molecule consists of a
short triple helix about 105 nm in length with a large globular domain
at each end.
Tendons often extend into the belly of a muscle or along its surface before
they merge with its connective tissue framework, and types I and III collagen
both may be extracted from meat. Even within tendons, there may be some
Type III collagen forming the endotendineum or fine sheath around bundles
of collagen fibrils. In fibers composed of collagen Types I and II, fibrils
have a straight arrangement whereas, in fibers of Type III collagen, the
fibrils have a helicoidal arrangement.
Small diameter type III collagen fibers are called reticular fibers
since, when stained with silver for light microscopy, they often appear
as a network or reticulum of fine fibers. The larger diameter collagen
fibers formed from Type I collagen are not blackened by silver.
Collagen fibers shrink when they are placed in hot water, and ultimately
they may be converted to gelatin. Around 65oC, the triple helix
is disrupted and the alpha chains fall into a random arrangement. The importance
of this change is that it tenderizes meat with a high connective tissue
content. Tropocollagen molecules from older animals are more resistant
to heat disruption than those from younger animals. In early studies, it
was suggested that reticular fibers, unlike collagen fibers, did not yield
gelatin when treated with moist heat. The original suggestion that reticular
fibers survive unchanged after cooking is wrong, but a modification of
the idea is plausible. Since a piece of meat may contain different types
of collagen, and since these types may differ in the thermal stability
of their cross links, it is possible that, at an intermediate level of
cooking around 65oC, endomysial collagen and perimysial collagen
may differ in the extent to which they are affected by the cooking treatment.
Heat-induced solubilization of Type I collagen is more important in improving
meat tenderness by cooking than is the effect of heat on Type III collagen.
Collagen biosynthesis
The synthesis of the different polypeptide strands that are combined to
make different types of collagen is genetically regulated by the production
of messenger RNA. The synthesis of polypeptide strands occurs on membrane-bounded
polysomes, but the hydroxylation of lysine and proline occurs after the
strands are assembled. Ascorbic acid is required for the hydroxylation
of lysine and proline. Polypeptide strands enter the cisternae of the endoplasmic
reticulum (a membraneous assembly labyrinth within the cell), the terminal
extensions of the strands are aligned, and then the strands spiral around
each other. Procollagen or immature collagen has long terminal extensions
protruding from each end of the newly formed triple helix. Procollagen
moves to the golgi apparatus and is packaged into vesicles that are moved
to the cell surface, probably by microtubules. Except for some Type III
procollagen molecules, the long terminal extensions are then enzymatically
reduced in length.
Outside the cell, collagen molecules become aligned in parallel formations,
and then they link up laterally to form fibrils. It is likely that tropocollagen
monomers are partially assembled together in groups before they are added
to an existing collagen fibril. Firstly, vacuoles containing procollagen
fuse to form a fibril-containing compartment. Then the cytoplasmic extensions
withdraw from between several fibril-forming compartments to create a bundle-forming
compartment. Sometimes collagen fibrils occur intracellularly, but it is
not clear whether this is collagen taken up by phagocytosis (engulfed by
the cell) or a surplus of newly synthesized collagen.
The characteristic parallel staggered arrangement of tropocollagen molecules
in a collagen fibril is caused by the 67 nm repeating pattern of oppositely
charged amino acids along the length of the tropocollagen molecule. The
degree of overlapping of adjacent molecules and the gaps left between the
ends of molecules cause the striated appearance of collagen fibers seen
by electron microscopy. The fibroblasts of young animals are metabolically
more active than those of older animals, particularly for aerobic metabolism.
Accumulation of collagen in meat
Although the relative proportions of Types I and III collagen in a muscle
may be related to meat tenderness, the overall amount of collagen and its
degree of crosslinking also are important. The absolute amount of collagen
in an animal may increase as animals become older, and this may have an
effect on meat toughness, but rapid growth of muscle fibers also may dilute
the relative amounts of collagen in meat. Considering the supposed importance
of collagen in meat toughness, the absence of overwhelming evidence from
taste panel studies is rather curious. It seems reasonable that stewing
beef is tougher than prime steak because it has more collagen, but is collagen
responsible for differences in tenderness between the same cut of steak
from different carcasses? Recent studies with UV fiber-optics suggest that
it is, because this new technology allows us to see overall trends that
are difficult to identify by chemical analysis of small samples of meat.
Within a carcass, there may be considerable differences in collagen
content between different muscles and this is reflected in their retail
price. Collagen content also may differ between sexes. For example, the
hydroxyproline content is higher in pork from females than castrated males.
However, the amount of collagen in meat, when expressed as a proportion
of wet sample weight, also is affected by fat content. In steaks from a
veal carcass, for example, the collagen content might exceed 0.5%, but
could be much less in the same region from a steer carcass in which fat
had accumulated to "dilute" the collagen content.
Collagen in meat may be studied by measuring collagen fibril diameters
in electron micrographs. In tendons, fibril diameters in the fetus are
unimodal but become bimodal in the adult. Large diameter fibrils may have
more intrafibrillar covalent cross-links, while small diameter fibrils
may have more interfibrillar non-covalent cross links. Thus, fibril diameter
may be related to fibril strength and elasticity. Meat with large diameter
collagen fibers tends to be tougher than meat with thinner collagen fibers.
Little is known about the mechanisms by which collagen fibers become
arranged in a muscle, or about the interactions which occur between fibroblasts
and the fibers that they produce, although it is possible that glycosaminoglycans
play some part in this interaction.
Collagen is very important in muscle development. Myoblasts, the cells
that form muscle fibers, develop a parallel alignment when cultured on
a substrate of Type I collagen, but they do not become elongated or aligned
on Type V basement membrane collagen. Myoblasts may themselves form Types
I, III and V collagen, while myotubes (immature muscle fibers) also may
form collagen, but only when associated with fibroblasts. The identification
of collagen in developing muscle is complicated by the fact that the tail
unit of the acetylcholinesterase molecule (involved in neural control of
muscle contraction) has a collagen-like sequence that contains hydroxyproline
and hydroxylysine.
Crosslinking of collagen molecules
Within an individual collagen molecule, the three polypeptide strands are
linked together by stable intramolecular bonds that originate in the non-helical
ends of the molecule.
The great strength of collagen fibers, however, originates mainly
from the stable intermolecular covalent bonds between adjacent tropocollagen
molecules.
Stable disulphide bonds between cystine molecules in the triple helix also
occur. During the growth and development of meat animals, covalent cross
links increase in number, and collagen fibers become progressively stronger.
Meat from older animals, therefore, tends to be tougher than meat from
the same region of carcasses from younger animals. This relationship is
complicated in young animals by the rapid synthesis of large amounts of
new collagen. New collagen has fewer cross links so that, if there is a
high proportion of new collagen, the mean degree of cross linking may be
low, even though all existing molecules are developing new cross links.
As the formation of new collagen slows down, the mean degree of cross linking
increases. Another complication is that many of the intermolecular cross
links in young animals are reducible (the collagen is strong but is fairly
soluble). In older animals, reducible cross links are probably converted
to non-reducible cross links (the collagen is strong but is far less soluble
and more resistant to moist heat). The chemistry of these changes is still
a subject for debate.
Pyridinoline, a non-reducible cross-link, may be involved in
the increased heat stability of epimysial connective tissues from older
animals. Although changes in collagen solubility might be an important
factor
affecting the tenderness of beef from older animals, the effect in younger
animals at a typical commercial slaughter weight may be relatively slight.
However, relative to increasing maturity levels used in US beef grading,
the pyridinoline content and thermal stability of intramuscular collagen
both increase.
Differences in the degree of cross linking may occur between different
muscles of the same carcass, and between the same muscle in different species.
For example, collagen from the longissimus dorsi is less cross-linked than
collagen from the semimembranosus, and collagen from the longissimus dorsi
of a pork carcass is less cross-linked than collagen from the bovine longissimus
dorsi. Nutritional factors such as high-carbohydrate diet, fructose instead
of glucose in the diet, low protein, and pre-slaughter feed restriction
may reduce the proportion of stable cross links. Nonenzymic glycosylation
(a reaction between lysine and reducing sugars) may be involved in the
interaction between diet and collagen strength. In general, the turnover
rate of collagen is accelerated in cattle fed a high energy diet. The rate
of collagen turnover in skeletal muscle may be about 10% per day and the
turnover time for collagen may be inversely proportional to collagen fibril
diameter.
Elastin and elastic fibers
Individual collagen fibers only lengthen by about 5% when stretched and
little elasticity is possible where collagen is formed into cable-like
tendons. However, much of the collagen that is present in meat forms a
meshwork so that stretching of the whole meshwork is possible because its
configuration changes. Fibers with truly elastic properties, however, are
necessary in structures such as the ligamentum nuchae of the neck and the
abdominal wall. And all arteries, from the aorta down to the finest microscopic
arterioles, rely on elastin fibers to accommodate the surge of blood from
contraction of the heart. Elastin fibers may be stretched to several times
their original length but rapidly resume their original length once released.
Elastin is found in all vertebrates except primitive jawless fish, and
in evolution it appeared first in cartilaginous fish. The elastin fibers
in the image below (from loose connective tissue around the intestine)
are the thin black ones. The much thicker, red-brown fibres are collagen.
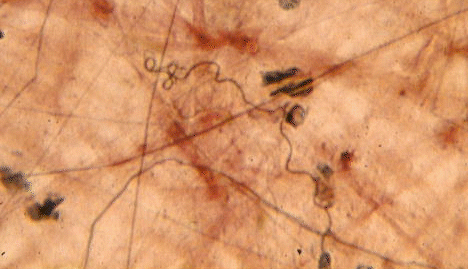
Elastic fibers are made of the protein elastin.
Elastin resists severe chemical conditions, such as the extremes of alkalinity,
acidity and heat that destroy collagen.
Fortunately, there are relatively few elastic fibers in muscle, otherwise
cooking would do little to reduce meat toughness. The elastin fibers in
muscles that are used frequently for locomotion are larger and more numerous
than those of less frequently used muscles. Elastin fibers in the epimysium
and perimysium of beef muscles range from 1 to 10 microns in diameter.
Elastin is synthesized by arterial smooth muscle cells, but the origin
of elastin in non-vascular locations is not properly understood. In the
lung, for example, large amounts of elastin are synthesized by various
types of lung cells but the cellular source of the elastin fibers in meat
is unclear at present. Some elastic fibers in muscle are involved in the
attachment of sensory organs called neuromuscular spindles.
Elastic fibers usually are pale yellow. When elastic fibers are stretched,
they may become visible in polarized light without staining, but this requires
careful attention to the refractive index of the mounting medium. In the
bovine ligamentum nuchae, the pattern of birefringence indicates that there
are two micellar structures, one arranged circularly on the outside and
the other arranged axially in the centers of the fibers. Elastic fibers
in meat have a small diameter (approximately 0.2 to 5 microns) although
they are much larger in the ligamentum nuchae. Elastic fibers in the connective
tissue framework of meat are usually branched.
Electron microscopy reveals that elastic fibers are composed of bundles
of small fibrils approximately 11 nm in diameter embedded in an amorphous
material. In the bovine ligamentum nuchae, fibrils may be constructed from
smaller units or filaments approximately 2.5 nm in diameter. Elastin filaments
are bound by non-covalent interactions to form a three-dimensional network
and elastic fibers are assembled in grooves on the fibroblast surface where
initially rope-like aggregations of fibrils become infiltrated with amorphous
elastin. Unlike the situation in elastic ligaments, where elastin forms
fibers, the elastin of the arterial system occurs in sheets that condense
extracellularly in the absence of fibrils.
Although elastin resembles tropocollagen in having a large amount of
glycine, it is distinguished by the presence of two unusual amino acids,
desmosine and isodesmosine. Like collagen, elastin contains hydroxyproline,
although it may not have the same function of stabilizing the molecule.
Tropoelastin, the soluble precursor molecule of elastin (molecular weight
70,000 to 75,000), is secreted by fibroblasts after it has been synthesized
by ribosomes of the rough endoplasmic reticulum and processed by the Golgi
apparatus. In the presence of copper, lysyl oxidase links together four
lysine molecules to form a desmosine molecule. Isodesmosine is the isomer
of desmosine. The aorta may be fatally weakened by a lack of mature elastin
in animals deprived of dietary copper. Elastin in the arterial system is
produced by smooth muscle cells instead of fibroblasts.
The functional properties of elastin in different tissues such as lung
and aorta may be related to differences in the ratio of tropoelastin A
to B. The elastin of elastic cartilage might be a different genetic type
to that found in the vascular system but, overall, the diversity of different
genetic types of elastin is far less than for collagen.
The cells of fibrous connective tissue
The dominant cell type in the fibrous connective tissue of meat is the
fibroblast, but other cells also exist. Macrophages or histiocytes are
sometimes quite numerous and, when inactive, may resemble fibroblasts in
appearance. However, the motility of macrophages is soon revealed by tissue
inflammation or the injection of colloidal dyes. Macrophages migrate through
the tissue and act as scavengers by engulfing invasive microorganisms or
foreign particles by phagocytosis.
Cells from the vascular system may wander through connective tissues
and even compact structures such as tendons have their own lymphatic and
vascular supply, something that is not easily seen in an exsanguinated
carcass. The vascular cells include a variety of lymphocytes and the plasma
cells responsible for antibody production. Eosinophils are cells with bilobed
nuclei and numerous cytoplasmic granules readily stained by eosin. The
skeletal muscles of cattle, and sometimes sheep, may become inundated with
eosinophils (eosinophilic myositis). The affected areas appear as irregular
pale lesions and often are detected by meat inspectors looking for muscle
parasites. Eosinophils may be attracted to areas of antibody activity and
eosinophilic myositis may be an allergic response.
Located around the body are some very interesting cells called mast
cells. They are involved in a variety of vital body functions, like resisting
disease, but they might also have a special importance for the meat industry.
Mast cells occur within the skeletal muscles of meat animals, mainly in
the perimysium and epimysium. The numbers of mast cells may be increased
in pathological situations and, in denervated muscle, mast cells may move
from the central tendon into the belly of the muscle. The cytoplasm of
mast cells contains large numbers of metachromatic granules (metachromasia
is a color change of dyes such as methylene blue so that metachromatic
granules are purple while the surrounding tissue is blue). Mast cells contain
heparin and histamine. Heparin prevents the coagulation of blood and histamine
increases the permeability of small blood vessels. Heparin also activates
the enzyme lipoprotein lipase involved in the accumulation of triglyceride
by adipose cells, so there could be some relationship between the distribution
of mast cells and the availability of fatty acids for storage in marbling
fat in meat. Mast cells also may release a substance that activates cell
division in nearby cells. Thus, in both availability of fatty acids for
storage and in the formation of new fat cells, the development of intramuscular
marbling fat in meat may have some relationship to the distribution of
mast cells. Mast cells sometimes come into close contact with skeletal
muscle fibers, but most mast cells are located along fine branches of the
lymphatic system in the perimysium and endomysium. Mast cells also have
been implicated in the regulation of collagenase activity and, thus, may
have part to play in the turnover of collagen and its cooking-resistant
strength.
Why meat must be a natural food for us
My favourite gem of information about connective tissue concerns the
digestibility of elastin. During the digestion of meat in the human gut,
elastic fibers are broken down by elastase, an enzyme from the pancreas
that would not be there if our evolutionary ancestors had not been at least
partly carnivorous. In other words, I have never read of the occurrence
of elastin in any human food except meat. So if we have evolved a highly
specific enzyme, elastase, to deal with elastin in our food, this can only
mean that we are the descendants of meat eaters.
The meat industry always seems to be under attack from the popular press
with a stream of bad news stories questioning meat in the human diet. Thus,
I derive great peace of mind from knowing scientifically that meat must
be a natural component of my diet. This fits nicely with my intuitive belief
that, thousands of years ago, my ancestors worked hard all day running
down something tasty to bring back to the family, and that the best part
of the day was sitting around a camp fire gnawing on a chunk of partly
burnt meat, chewing the fat, and washing it down with home-brew.
�
 The fibrous connective
tissues in meat form a continuous mesh, as shown in the image to the left,
from the microscopic strands of endomysium around individual muscle fibers,
to the larger layers of perimysium that delineate bundles of muscle fibers,
all being gathered and connected to the thick, strong epimysium on the
surfaces of individual muscles.
The fibrous connective
tissues in meat form a continuous mesh, as shown in the image to the left,
from the microscopic strands of endomysium around individual muscle fibers,
to the larger layers of perimysium that delineate bundles of muscle fibers,
all being gathered and connected to the thick, strong epimysium on the
surfaces of individual muscles.


 Collagen fibrils
are formed from long tropocollagen molecules which are staggered in arrangement
but tightly bound laterally by covalent chemical bonds. For electron microscopy,
when negatively stained with heavy metals that spread into the spaces between
the ends of molecules, collagen fibrils appear to be transversely striated.
The periodicity of these striations is 67 nm but often shrinks to 64 nm
as samples are processed for examination. Although collagen fibers are
located outside the cell, the initial stages of collagen fibril
assembly may be within the cell, with fibril morphology being regulated
by a special site on the fibroblast membrane (cells that form connective
tissue fibers are called fibroblasts).
Collagen fibrils
are formed from long tropocollagen molecules which are staggered in arrangement
but tightly bound laterally by covalent chemical bonds. For electron microscopy,
when negatively stained with heavy metals that spread into the spaces between
the ends of molecules, collagen fibrils appear to be transversely striated.
The periodicity of these striations is 67 nm but often shrinks to 64 nm
as samples are processed for examination. Although collagen fibers are
located outside the cell, the initial stages of collagen fibril
assembly may be within the cell, with fibril morphology being regulated
by a special site on the fibroblast membrane (cells that form connective
tissue fibers are called fibroblasts).
