8 Fibre Types
8.1 Introduction
- The last lecture
explained how myoblasts fuse together to form multinuclear myofibres,
with the oldest myofibres passing through a myotube stage of
development and the youngest myofibres passing through a secondary
fibre stage of development.
- The formation of myofibres from mesodermal
cells through a series of transitional cell types (premyoblast,
myoblast and myotube or secondary fibre) is a classical example
of cellular differentiation.
- Cellular differentiation leads to an efficient
and mutually advantageous division of labour among the tissues and
organs
of the body.
- In skeletal muscles, physiological
differentiation continues after
the myofibres
have been formed and have reached a functional state.
- Physiological differentiation creates populations of
fast- and slow-contracting myofibres with appropriate sources of
energy for contraction, either aerobic (using blood-borne oxygen
for complete oxidation of
substrates) or anaerobic
(incomplete oxidation of carbohydrates without need
for oxygen).
- A key point to remember is secondary fibres developed around
myotubes - so myotubes are located centrally within a fasciculus
(bundle of myofibres).
8.2 Red and White Muscle
Red and white muscle is a concept widely used by both
meat traders and consumers. An obvious example is the comparison
of breast meat with leg meat in chickens. The breast meat is
white. The leg meat is red. Another example is seen in
whole cross sections through a cured ham. The outer muscles are
less pigmented than deep muscles around the bones. Although
not as clearly different as in the chicken, the less pigmented
superficial ham muscles are called white muscles and the heavily
pigmented deep ham muscles are called red muscles. Finally, we often
use the term white meat for all types of chicken and veal, in contrast
to red meats such as beef and lamb. A little confusing, eh? Keep in
mind these two points.
- Each of our meat animals has some degree of physiological
differentiation among muscles. Fast-contracting muscles are
needed for locomotion. Slow-contracting muscles are needed for
respiration, chewing and maintaining posture.
- There are differences among our species of meat animals in
their overall degree of muscle pigmentation. Beef is darker than lamb,
lamb is darker than pork, and pork is darker than chicken or turkey.
Consider these two points together. We will only SEE differences between red and
white muscles when the overall degree of species pigmentation is medium
(pork) or low (chicken). Physiological differentiation certainly exists
in beef - there are both fast- and slow-contracting muscles - but we
cannot easily SEE the
differences.
The final point - IT IS THE
MYOFIBRES WHICH ARE PHYSIOLOGICALLY DIFFERENTIATED. Nearly
all muscles in our meat animals have a balance of fast- and
slow-contracting myofibres. If most myofibres in a muscle are
fast-contracting, then the overall contraction speed of the muscle is
fast (a white muscle). If most myofibres in a muscle are
slow-contracting, then the overall contraction speed of the muscle is
slow (a red muscle).
8.3 Myoglobin
.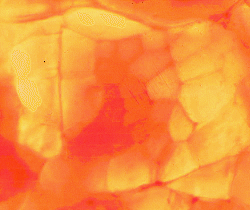
The image above shows a thin slice of pork illuminated from below
and viewed with a low-power microscope. A fasciculus fills most of the
image. Individual myofibres are clearly visible. The central
myofibres are heavily pigmented with myoglobin.
The surrounding myofibres have very little myoglobin. This is a white
muscle. Most myofibres have a low concentration of
myoglobin. If we attempted the same demonstration with white
chicken breast meat - we would see no heavily pigmented myofibres. If
we attempted the same demonstration with any beef muscle - we would see
all myofibres heavily pigmented.
Myoglobin is very soluble and is located inside myofibres
in living animals. But fluid leaks from myofibres in meat, and so
myoglobin colours the juices lost from meat in the supermarket.
Haemoglobin is the red pigment
within red blood cells (erythrocytes).
Meat animals are exsanguinated at slaughter. THERE IS NO
HAEMOGLOBIN IN MEAT. The fluid leaking from meat does not contain
any blood. The fluid leaking from meat does not contain any
haemoglobin. The only traces of haemoglobin we sometimes find in
meat or meat juices come from a few erythrocytes remaining in
capillaries. The wiggly blue lines in the image below are capillaries
running on the surfaces of myofibres in beef (they have been stained
with methylene blue dye).

- It is important to remember, in
the living animal, oxygen is brought to the surfaces of
myofibres by haemoglobin in erythrocytes. From here, the transport of
oxygen to the interior of the myofibre is facilitated
by myoglobin. Thus, myofibres specialized for aerobic metabolism
develop a
high myoglobin concentration. Diving mammals may develop very high
levels of myoglobin to store oxygen - much higher
than the levels of myoglobin used to facilitate oxygen transport in our
meat animals.
- The dominant work of some muscles is to maintain a standing
posture
or to contract slowly during locomotion, chewing or breathing. These
muscles contain a high proportion of slow-contracting and
fatigue-resistant myofibres with a high myoglobin concentration. Thus,
the capillary bed of red muscles
is more dense than in white muscles.
8.4 Fast and Slow Fibres
Historically, it appeared the relationship
between fast- and slow-contracting myofibres in animals was quite
simple. The subject really got started in 1873 with the great
French histologist, Ranvier (who gave his name to the nodes of
Ranvier along myelinated axons). It became accepted fast
myofibres were usually
white (low myoglobin), while slow myofibres were usually red (high
myoglobin). When redness was found to be
due to myoglobin, and myoglobin was found to be correlated with aerobic
metabolism, this explained the relationship between redness and speed
of
contraction. The pale or white myofibres with a low aerobic potential
were
found to be well endowed with glycolytic enzymes enabling them to
obtain
energy rapidly by the incomplete oxidation of glycogen.
This explained why white myofibres soon became fatigued once their
glycogen
stores were depleted and why they had to wait for the removal of
lactate
by the circulatory system.
At the extremes of the range in physiological differentiation
(fast white myofibres versus slow red myofibres) these discoveries are
still
valid. The problem, as we see it now, is there are also
myofibres
with a fast contraction speed and a dual energy supply. In other
words, some fast myofibres have both aerobic and anaerobic capabilities.
The discovery of these myofibres with dual energy supply coincided in
a most confusing way with a
growing awarenesss slow red myofibres in meat animals and poultry
were
rather different from those of frogs and other animals used in
biomedical research. It is difficult to write a research report
on myofibre types without giving them names. Unfortunately, everybody
seemed to use different names, and the numbers of myofibre types
recognized tended to be a function of the number of techniques
used to identify them.
Cutting a long story short, most researchers recognize three
main types of myofibres (each with multiple names).
- Red = beta-R = Type I,
distinguished by histochemical features indicative
of a slow contraction speed (e.g.., acid-stable ATPase, alkali-labile
ATPase)
plus features indicative of strong aerobic metabolism (eg., strong
mitochondrial
SDH activity).
- Intermediate = alpha-R = Type II
red, distinguished by features indicative
of a fast contraction speed (eg., acid-labile, alkali-stable ATPase)
plus
features indicative of strong aerobic metabolism.
- White = alpha-W = Type II white,
distinguished by features indicative of
a fast contraction speed plus features indicative of weak aerobic
metabolism
(eg., low SDH activity).
8.5 Histochemical reactions
ATPase reaction.

A frozen section of muscle is exposed to ATP solution and ATPase within
myofibres
cleaves off the phosphate. But the phosphate is invisible and tends to
move around. First we stop the reaction product moving by precipitating
the phosphate with
cobalt, then we blacken the cobalt salt
by converting it to a sulphide. If that is all we do, all the myofibres
will
go black, because they all have ATPase. So first of all, before
starting the reactions described above, we pre-incubate the frozen
sections of meat in solutions (such as acetic acid, formaldehyde, etc)
to inactivate the isoenzyme
in either the fast or slow-contracting myofibres. Then we see
differences between
myofibres, as above. This is a section of pork. The fast-contracting
myofibres are located peripherally in their fasciculi. Therefore, we
are looking at ATPase activity in fast-contracting myofibres which was
stable when the section was pre-incubated with formaldehyde. The
unstained myofibres are slow-contracting fibres whose ATPase was
inactivated by the formaldehdye. Incubation with acetic acid
instead of formaldehdye would have produced the opposite staining
pattern.
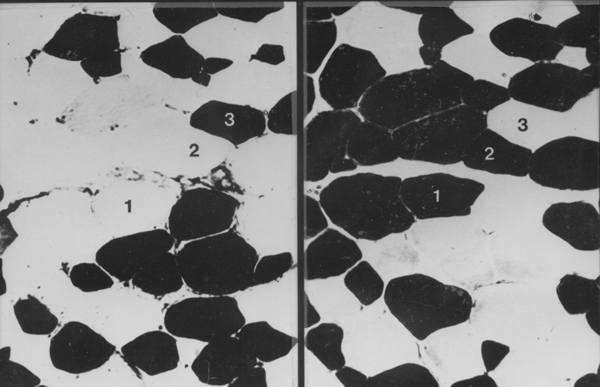
In the example above, 1 is a white myofibre (alpha-W or Type II white),
2 is an intermediate myofibre (alpha-R or Type II red) and 3 is a
red myofibre (Beta-R or Type I). The section on the left was
pre-incubated with acetic acid. The section on the right was
pre-incubated with fomaldehyde. How did I separate myofibres 2 and 3
which both have the same ATPase reaction? Answer - using the SDH
reaction on a serial slice.
SDH reaction.
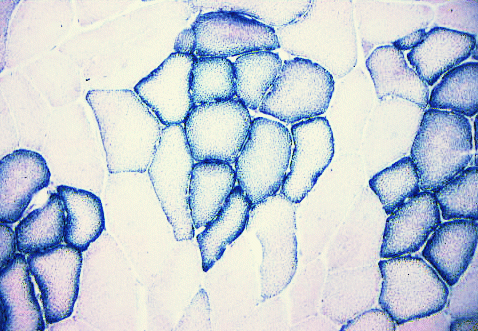
SDH (succinate dehydrogenase) is an enzyme specific to
mitochondria.
Each little granule of diformazan (the reaction product of nitroblue
tetrazolium)
indicates the location of mitochondria. This is a section of
pork. The aerobic myofibres are grouped centrally within their
fasciculi surrounded by anaerobic myofibres. Note how SDH is
concentrated under the cell membrane around the outside of the
myofibre.
Phosphorylase reaction.

Phosphorylase
is the first enzyme involved in glycogenolysis.
It normally breaks down
glycogen, but we can make it run backwards to make new
glycogen (amylose) stainable with iodine (the
reaction works best if there is some natural glycogen present in the
myofibre to start the reaction). Thus, absence of a phosphorylase
reaction
does not automatically mean that there is no phosphorylase present!
Stain for triglyceride - Sudan Black B.
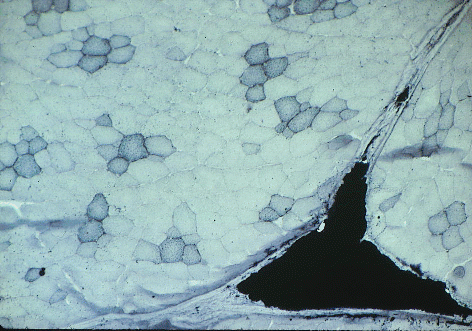
Sudan black has stained the lipid
droplets inside
aerobic myofibres in this slice of pork, and it has also stained a
large
triangle of intramuscular (marbling) adipose cells between the muscle
fasciculi.
8.6 Aerobic versus anaerobic features
Many of the cellular features associated with aerobic and anaerobic
metabolism in myofibres are fairly straightforward.
Aerobic myofibres
are:-
- served by a more dense capillary meshwork than fibers with a poor
aerobic
potential;
- their sarcoplasm contains more mitochondria and more lipid
droplets; and
- the enzymes involved in aerobic metabolism are more concentrated.
Quantitatively, however, the range from aerobic to anaerobic metabolism
is usually a continuous variable and is seldom broken into
discontinuous
steps.
From which we may deduce two points:
- Firstly, by manipulating the pH of ATPase incubation media or by
using antibody reactions, it is
possible
to generate more than two staining responses for ATPase (i.e., more
than just fast- versus slow-contracting), and these do not fit very
well with the categories of just three histochemical types.
- Secondly, there is evidence the physiological differentiation of
myofibres is a dynamic balance in the division of labour, and that the balance
may change during growth or in response to a change in the work pattern
of a muscle.
Thus, to some researchers, the histochemical categorization of
myofibres by any method, including myofibrillar ATPase, is merely a
useful, but artificial
subdivision of a continuously variable range. Myofibres may
undergo a continual alteration throughout life as an adaptation
to changing functional demands, and "fibre type" merely reflects the
constitution of a myofibre at any particular time. From an agricultural
viewpoint, this is particularly interesting
since it suggests the existence of some degree of genetic or
developmental
plasticity in the fibre type continuum. In meat animals, this might be
a vital link in relating muscle growth to meat quality.
8.7 Intracellular differentiation
Physiological differentiation may vary intracellularly across
individual myofibres, at least as far as aerobic metabolism
is concerned. But as far as is known at present, factors relating to
contraction speed are fairly uniform within individual myofibres.
Aerobic
metabolism, as indicated by the distribution of mitochondria, may be
graduated
radially so that the subsarcolemmal region (the outer part of a
myofibre) has a high level of aerobic
metabolism while the central axis has a low level. Mitochondria from
peripheral
and axial regions of the myofibre may differ in their biochemical
characteristics,
and. proportional mitochondrial volume and maximal rate of oxygen
consumption
are linearly related among different muscle regions.
The subsarcolemmal concentration of mitochondria in some types of
myofibres may be related to the supply of oxygen arriving in
capillaries on the surface of the
myofibre. Mitochondria are larger in red myofibres than in intermediate
or white myofibres and, in red myofibres, they may form thick
longitudinal
columns
between the myofibrils. The arterial and venous elements of muscle
capillaries
tend to occur in an alternating manner along the length of the
myofibre, with
longer arterial segments of capillaries in white muscle relative to red
muscle.
 This image shows the results of computer mapping of the
SDH deposits in a myofibre. Dark blue shows high SDH, and light blue
shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying
the
radial gradients of SDH activity in different types of meat
animals.
This image shows the results of computer mapping of the
SDH deposits in a myofibre. Dark blue shows high SDH, and light blue
shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying
the
radial gradients of SDH activity in different types of meat
animals.
Further information
Structure and Development of Meat
Animals and Poultry. Chapter 6.
Trivia
The microgaphs above are from my own research from 1970 to 1990. The
last image, computer mapping of diformazan, dates from the early
1980s. This was one of the first colour screens easily available
(Intecolor 3650). The pixels were controlled with 8-bit assembler.








 This image shows the results of computer mapping of the
SDH deposits in a myofibre. Dark blue shows high SDH, and light blue
shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying
the
radial gradients of SDH activity in different types of meat
animals.
This image shows the results of computer mapping of the
SDH deposits in a myofibre. Dark blue shows high SDH, and light blue
shows low SDH (and cyan
is medium). Once on the computer, these data can be used for studying
the
radial gradients of SDH activity in different types of meat
animals.