7 Myogenesis
7.1 Introduction
Myogenesis
is the creation of muscle tissue from stem cells in
the embryo. Muscle becomes meat, and we are interested in its early
origin. Some animals produce high-quality meat, others produce it very
rapidly. Can one animal do both?
Bulk meat, such as a steak or
roast, is composed of countless
microscopic muscle fibres (myofibres). Each myofibre is multinucleate
(has numerous
nuclei) because the myofibres are very long (usually many centimetres).
Thus, one nucleus could not possibly produce enough RNA for
protein synthesis in the whole myofibre. How does this
multinucleate
condition arise?
The components involved are as follows.
- Premyoblasts - cells
capable of mitosis but not yet producing muscle proteins.
- Myoblasts - cells no
longer capable of mitosis but now starting to produce muscle proteins.
- Myotube - a
multinuclear myofibre produced by the fusion of myoblasts.
- Secondary fibre - a
multinuclear myofibre produced by the fusion of myoblasts on the surfaces of a myotube.
- Myofibre - a muscle fibre matured
from either a myotube or a secondary fibre.
7.2 Mitosis
Mitosis is cell division. First
the nucleus doubles its DNA, then it divides. Then the rest of the cell
divides to produce two identical daughter cells. The mesodermal cells
of somites and limb buds undergo frequent mitosis,
with a variety of factors such as IGF-1 (insulin-like growth
factor) and PDGF (platelet-derived growth factor - platelets
are cell fragments in the blood stream) being
mitogenic (causing mitosis). The peak of mitotic activity in the limb
buds of the chick
embryo is at about 5 days incubation - we would love to have similar
information on cattle, sheep and pigs.
Dividing premyoblasts are rounded in
shape (but compressed together) and are locked into a mitotic cycle.
- G1, (gap one) or rest after the last mitosis (2.0
hr)
- S, synthesis of new DNA (4.3 hr)
- G2, (gap two) or rest after DNA synthesis (2.4 hr)
- M, mitosis (0.8 hr)
- return to G1 or become a myoblast (5-7 hr)
The times given are approximations for premyoblasts growing in the
laboratory. They give us a guess of how long these
events might take in farm animals. The escape from this cycle - when a
premyoblast becomes a postmitotic
myoblast - is thought to be irreversible. The cycle preceding a
premyoblasts's
escape has been termed the quantal division. The
number of times a clone of premyoblasts remains locked into the
mitotic
cycle might have a profound importance on myoblast numbers. Just one
extra cycle by all premyoblasts might double the number of myoblasts
and give
rise to extra myofibres (hyperplasia).
The population of
premyoblasts capable of mitosis may not be completely homogeneous since
it might contain true stem cells and committed precursors. A committed
precursor is a cell giving rise to a cohort of 16 terminally
differentiated myoblasts. Obviously, factors regulating
premyoblast proliferation, such as triiodothyronine (a hormone
produced by the thyroid gland and otherwise associated with heat
regulation in the body), are extremely
important to the meat industry.
Another way of looking at this system of cell proliferation is to
consider premyoblasts at the escape point in their mitotic cycle. Both
the
daughter cells produced by mitosis may stay in the cycle, both may
escape to become myoblasts, or one may stay in and one may escape. With
a population of cells, the percentage of escaping cells starts at 0% in
very young embryos, before the appearance of any myoblasts, and then
increases towards, but never reaches
100% (some stem cells remain as satellite
cells, a source of muscle nuclei during growth and regeneration).
Cell populations containing mixtures of premyoblast stem
cells,
mononucleate myoblasts and fused myoblasts can be sorted with arabinocytidine.
This prevents the formation of new myoblasts but does allow cell
fusion. In cultures from 11-day chick embryos, about 20% of cells are
myoblasts, but the percentage is lower in younger embryos. Another way
of sorting cells is to determine what percentage may be cloned to give
rise to myoblasts capable of fusion. Chick leg bud mesoderm at 72 hours
incubation contains 0%, at 80 hours it contains 10%, and at 6 days it
reaches 60%. In human limb buds, comparable values are 14% at 36 days,
with a 90% plateau from 100 to 172 days.
Another factor controlling cell proliferation might be the duration
of the mitotic cycle, possibly by a variation of the duration of G1
. Premyoblasts escaped from the mitotic cycle to become myoblasts
eventually fuse together, but the fusion of cells eventually becomes
less frequent, as if inhibited. Alternatively, escape from the mitotic
cycles may be in late G1. Cells in G1 may respond to PROSTAGLANDIN
E1 with a transient increase in intracellular cyclic AMP.
This may activate protein kinase and the onset of myoblast fusion.
The nervous system exerts some regulation over
muscle development, and its control over myoblast proliferation is
probably achieved by varying the duration of G1 rather than G2.
Because of the importance of G1 in the regulation of cell numbers, it
is interesting to note the G1 -S boundary is the point at which
the cell synthesizes calmodulin. Calmodulin is a protein able to
bind
calcium ions, and is thought to be involved together with cyclic
AMP in the regulation of many aspects of cell metabolism, growth and
division.
7.3 Myoblasts
The morphological features of premyoblasts are similar to those of
other types of precursor cells
in the embryo. RNA synthesis dominates cell activity and results in a
large oval-shaped nucleus, prominent
nucleoli (which vary in number between
species), diffuse chromatin (nuclear DNA) and many ribosomes (granules in the cytoplasm
responsible for protein synthesis). The large amount of RNA (an acid) in the cytoplasm binds
to basic (alkaline) dyes, and the cytoplasm is described as basophilic (base-loving). Myoblasts
are bipolar,
spindle-shaped cells, whereas fibroblasts tend to be triangular in
shape. Myoblasts may form tight junctions where they are in contact
with each other, usually at the tips of their elongated cytoplasmic
extensions. Here we see myoblasts fusing and becoming lined up.
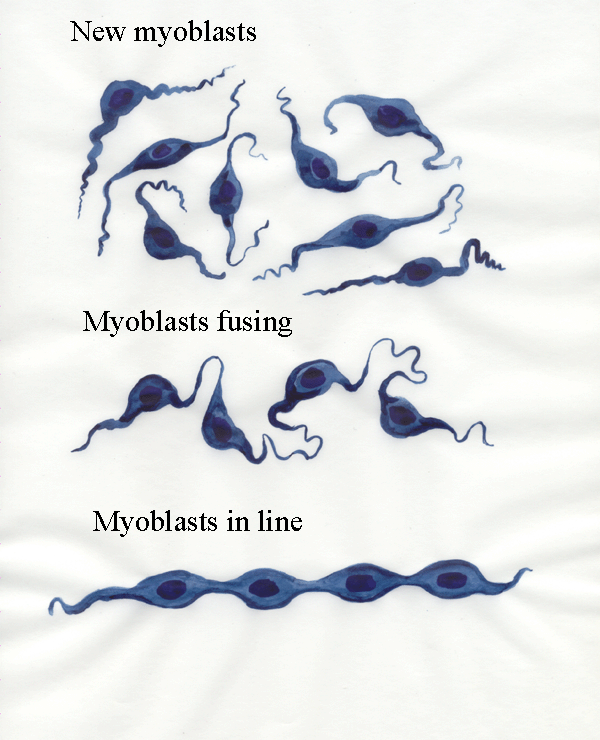
The process of lining up is very important. It can only occur if the
free end of the myoblast at one end of the line attaches itself to an
appropriate point at one end of the future muscle and if the free end of the myoblast
at the other end of the line attaches itself to the other end of the future muscle. This
brings the line of myoblasts into line with the long axis of the
muscle. The mechanism may be myoblasts following the lines of
connective tissue fibres in the developing muscle (contact guidance). If bad
connections are made (say both ends of the line of myoblasts attach to
the same end of the muscle) - then the line of myoblasts degenerates.
Thus, a developing muscle contains many degenerating myoblasts which
have failed to develop appropriate connections. Only if the line
of myoblasts is properly attached at both ends can the myoblasts
contract, stretch their membranes, and take up amino acids for further
protein synthesis.
Fusion is preceeded by a period of cell to cell recognition in which
the myoblasts may still be dispersed chemically with EDTA (which
removes calcium ions and loosens cell contact). Recognition is
followed by a period of adhesion in which trypsin (an ezyme able to
attack proteins) must be added
experimentally in order to disperse the cells. Finally, after membrane
fusion, fused cells cannot be dispersed. Cultured myoblasts fuse when
their numbers reach a certain density, perhaps in response to a
chemical signal. Within the myoblast, an increase in the level of cyclic
AMP initiates the events that lead to fusion. Myoblasts have
surface antigens for cell-cell recognition.
Myoblast fusion is triggered by calcium ions but is inhibited by
magnesium and potassium ions.
7.4 Myotubes
This simple explanation only holds true for muscles with a simple
structure - parallel myofibres running from one end of the muscle to
the
other. Most muscles in meat animals have a complex structure with
an angular arrangment of myofibres onto a tendon at one of the
muscle. Thus, in most muscles, development occurs in subunits (in
bundles of myofibres called fasciculi
- the singular is fasciculus).
Within each fasciculus,
therefore, lines of myoblasts develop running from one end to the other.
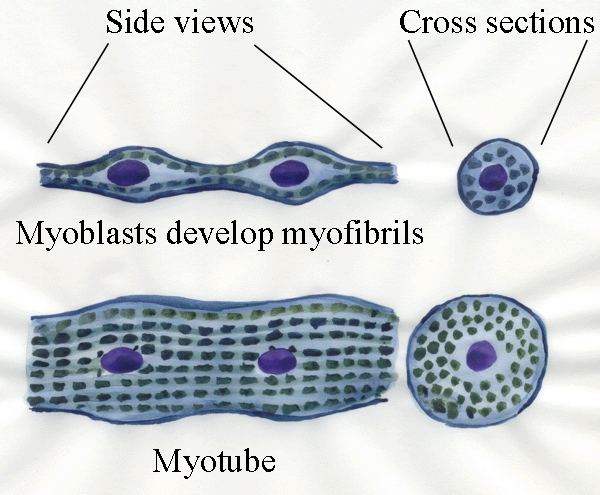
Next, the myotubes start to develop myofibrils.
Much more will be said about myofibrils later - here we only need to
know they are responsible for muscle contraction and they run
longitudinally with their striations in line across the future
myofibre.
Myofibrils are added around the nuclei and the nuclei remain in
the long axis. In the early days of microscopy, the nuclei were
difficult to see (because they are only easily visible if they are
stained, and appropriate stains had not yet been invented). Thus, early
microscopists saw only tubular structures (formed by the myofibrils)
and named them myotubes.
7.5 Secondary fibres
Getting myotubes properly lined up in the future muscles takes a long
time - and the time for parturition (birth) is rapidly approaching. The
muscles have only about 20% of their future myofibres formed by these
myotubes. What next? A very rapid process of forming secondary fibres
from a new generation of myoblasts.

New myoblasts take advantage of the myotubes being properly lined up in
the approriate pattern for the future muscle. The new myoblasts
attach themselves to the myotube surface and, when the myotube
contracts, it brings the myoblasts into the correct alignment for
fusion. The fused myoblasts now become a secondary fibre as they start to
produce myofibrils. But the secondary fibre is not yet innervated - it
does not contract next time the myotube contracts. So the secondary
fibre shears off from the surface of the myotube once it is
sufficiently stiffened by its new myofibrils. In this remarkable
process of mass production we see 80% of the future myofibres of
our meat animals being formed very rapidly just before birth.
7.6 Implications
- 80% of our meat comes from secondary fibres.
- Anything inhibiting myotube contraction will reduce the numbers
of future myofibres (for example, loss of amniotic fluid preventing
limb movements by the foetus or environmental toxins affecting
neuromuscular excitation).
- Although both myotubes and secondary fibres go on to become
myofibres, they will often become different types of myofibres.
Often the myotubes will become slow-contracting myofibres used for
fatigue-resistant contraction while secondary fibres will become
fast-contracting myofibres used for bursts of strong muscle contraction
(which are easily fatigued). Slow-contracting myofibres give meat much
of its taste and succulence. Fast-contracting myofibres account
for much of the rapid muscle growth in meat animals. Obviously,
there are many complexities to add to this simple but true
generalization - these will be explained later.
- An
example of the importance of muscle innervation in meat
production!
7.7 Commitment, differentation & maturation
The overall sequence of events in myogenesis may be separated into
commitment, differentiation and maturation.
- Commitment occurs when a
stem celll has its
future restricted to myogenesis.
- Dfferentiation is marked
by the transcription of genes coding
for typical features of the myofibre.
- Maturation or terminal
differentiation occurs after innervation.
Myogenin and MyoD are genes in a family activated when
commitment to a myogenic lineage occurs. These genes could be very
useful in
exploring
the factors determining muscle size in meat animals. Myogenin and
MyoD are sensitive to thyroid hormones, as well as being regulated by
muscle electrical activity, possibly via a mechanism dependent on
cyclic-AMP. Innervation controls the abundance of myogenic factors such
as MyoD1 and myogenin, and denervated muscle reverts to a neonatal
state (that is, cut the nerve to a muscle, and the muscle may revert to
the
state it had before it was first innervated). Subject to neural
regulation, MyoD is prevalent in fast
muscles, and myogenin in slow muscles.
Transforming growth factor beta 1 (TGF-ß1)
is a small
peptide involved the joint develop of myofibres and connective
tissues. Following local induction of TGF-ß1, it may produce local
gradients enhancing the development of connective tissues by
fibroblasts, but inhibiting myogenesis. Thus, a reduction of TGF-ß1
gradients might produce a condition similar to that found in
double-muscled cattle.
7.8 Myofibre arrangement
- The major nerve trunks grow into a limb bud by following
the
connective tissue framework of the bud, but developing muscles with
more than about 10 myotubes may be
necessary to invoke the formation of side branches of nerves to the
muscle.
- Muscles may be attached to either the shaft (diaphysis) or the
knob
(epiphysis) of a bone. But the longitudinal growth of bones occurs at
cartilagenous epiphyseal plates, and one of these plates is located
between each epiphysis and its diaphysis. Thus, to retain their
positions relative to each other during epiphyseal plate growth, some
muscle attachments must migrate
over the bone surface. Muscle
migrations are regulated by the bone and traction by the periosteum
(the membrane around the bone) is
responsible for the migration of tendon insertions.
- Muscle development
in the limbs of foetal meat animals may be shaped by a dynamic
interaction
between linear skeletal growth and the resistance of muscles to
stretching.
- If muscle stretching shapes muscle growth, the
determination of myofibre arrangment might be explained by the
contact guidance theory attempting to explain how nerve cells invade
developing tissues. Myotubes and myoblasts might be guided by a matrix
of very fine connective tissue fibres. Migrating myogenic stem cells in
chick embryos branch into filopodia at
their leading edges, and stem
cells follow the alignment of fine connective tissue fibres. The ends
of myotubes actively grow through the tissue of the future muscle and
have a well developed cytoskeleton dominated by microtubules.
- Molecules of fibronectin
have binding sites for a number of the
components surrounding cells (such as for collagen and
glycosaminoglycans) but also they can bind to the surfaces of cells.
Thus, matrices of fibronectin may be involved in the guiding of cell
migrations and the determination of muscle architecture.
- The initial
arrangement of connective tissue fibres is probably determined by
fibroblasts stretching the extracellular matrix.
- Cultured myoblasts only develop a parallel alignment if they are
cultured on a type of collagen able to form distinct collagen fibres.
- Myotubes may be pulled into alignment by their already
anchored
ends to follow the dominant directions of a stretched matrix.
- The angular arrangement of myofibres is difficult to explain.
Perhaps the tensile forces shaping the connective tissue matrix of a
pennate muscle are transmitted by intramuscular tendons. Another
possibility is myoblast arrangement may be influenced by the orientation
of electrical fields. Cultured myoblasts become arranged with their
long axes perpendicular to electric fields of 36 to 170 mV/cm.
- Intracellularly, the parallel arrangement of myofibrils
is dependent
on the proper attachment of the whole myotube or secondary
fibre. New filamentous proteins for incorporation into myofibrils
appear
first at the periphery of cells - thus, the longitudinal
orientation of filaments may follow the direction of membrane
stretching.
7.9 Degeneration and survival
Many of the early histologists who studied myogenesis were impressed
by the widespread evidence of cellular degeneration they
found in developing muscles. Lysosomes (membranous bags full of deadly
digestive enzymes - often called suicide bags) capable
of causing degeneration are well developed even in myoblasts.
Experimentally, if myofibres are slowly stretched they will continue to
develop. But they degenerate if they are not stretched. The passive
stretching of myotubes
activates the sodium ion pump of their membranes, and this is followed
by increases in amino acid uptake and protein synthesis. The
stimulation of amino acid
transport and protein synthesis induced by the stretching of myotubes
may act through the
release of messenger substances such as arachidonic acid,
diacylglycerol and prostaglandins.
This is a very important point concerning muscle growth - not just
in meat animals, but in ourselves as well. We all know exercise
encourages muscle development while inactivity allows muscles to waste
away. The mechanism involves cell membranes. When myotubes or
secondary fibres get properly attached at their ends, they can
contract. When they can contract, they can stretch their cell
membranes. When their cell membranes are stretched, the uptake
of amino acids is enhanced.
Further information
Structure and Development of Meat
Animals and Poultry. Chapter 6.


