23 Radial Growth of Muscle Fibres
23.1 Introduction
The basic concepts are
relatively simple. Animals with bulging muscles have a high yield of
meat. Muscles are composed of myofibres, and when these grow radially- so does the whole
muscle. Here we will be
concerned with how myofibres grow radially, and with complexities
of muscle structure which complicate things .
-
The radial growth of myofibres is an example of cellular hypertrophy - the myofibre (a giant
cell) is growing in size.
-
But when the myofibre undergoes hypertrophy - it also
increases its number of myofibrils.
-
Thus, myofibre hypertrophy is accompanied by myofibrillar hyperplasia (increase in number -
not size).
-
Longitudinal growth of myofibres differs from radial
growth (although both are examples of cellular hypertrophy), and is
examined in the next lecture.
23.2 Myofibre diameters and myofibrillar hyperplasia
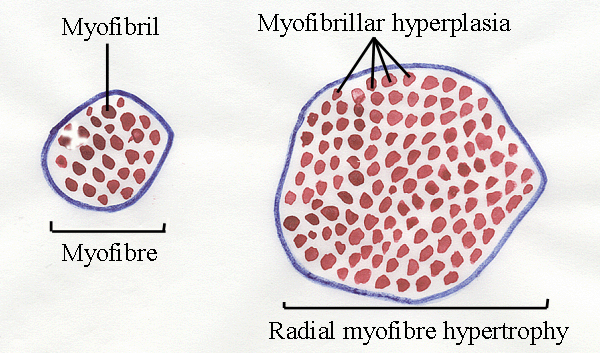
-
As a myofibre
grows in diameter, there is an
increase in the number of myofibrils.
-
At one time, it was suggested myofibre
diameters might be used to measure muscularity in meat animals, but
hopes of this faded rapidly once the complexity of the interactions
between
myofibre number, length and diameter became known.
-
Myofibre diameters are negatively correlated
with the tenderness of cooked meat, but this may not be a direct
relationship. For example, as animals grow older, their myofibres
increase in diameter, but they also develop stronger and more abundant
connective tissue. Thus, the real relationship may be between
connective tissue and tenderness.
-
In young animals, fast-contracting myofibres usually
have more rapid radial growth than slow-contracting myofibres.
-
In older animals, radial growth of fast-contracting
myofibres slows down, but radial growth of slow-contracting myofibres
continues. This is because the slow myofibres are primarily
involved in the maintenance of body posture against gravity - and the
animals are now getting very heavy.
-
All other things being comparable, animals on a low plane of nutrition will
have smaller myofibre
diameters than animals on a high plane of nutrition.
-
All other things being comparable, entire males will have larger diameter myofibres
than females. This may be detectable to the consumer as coarse meat texture.
-
Measurement of radial growth is difficult because
myofibres are not really cylindrical - they tend to be prismatic - with flat sides where
they are pressed together. Where would you measure the diameter
of the myofibre shown above?
-
Another problem is myofibres are often tapered -
their diameters may decrease towards one or both ends of the myofibre.
-
And, of course, myofibre diameters increase when a
myofibres contracts! Seldom do researchers correct
for differences in sarcomere length.
Formation of new myofibrils
- New myofilaments are added around the outside of the myofibril.
- This increases the length of the diffusion pathway for calcium ions as they turn
contraction on and off. (Remember
- calcium ions are released from the sarcoplasmic reticulum to
initiate muscle contraction, then re-sequestered for relaxation).
- This causes mechanical stress in the myofibril - the outside contracts and relaxes before the
central axis.
- Calcium ions building up in the interior of the myofibril
activate enzymes (calpains)
which release thin myofilaments from their Z-lines.
- Contraction causes the weakened myofibril to split.
- But, the myofibril is very long, and a split at one point
may not match up to a split at another point.
- We end up with a complex structure. Where once there
was a single myofibril seen in transverse section, we now see many
myofibrils in transverse section - but following them along the
myofibril we find they are all linked.
- Thus, we have not really
formed any new myofibrils, just increased their size and complexity.
- But, it appears we have formed new myofibrils, so we will
keep on talking about their numbers!
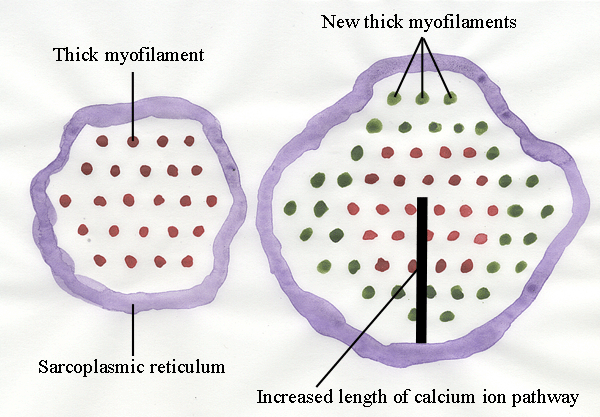
To keep things simple in the diagram above - we are looking at
transverse sections at the midlength of relaxed sarcomeres where we
will only see thick myofilaments. The new new myofilaments are shown in
green. You can see how the progressive addition of new myofilaments
will increase the length of the pathway by which calcium ions move in
and out of the myofibril.

23.3 New myofibre nuclei
As the myofibre grows radially, it adds new nuclei.
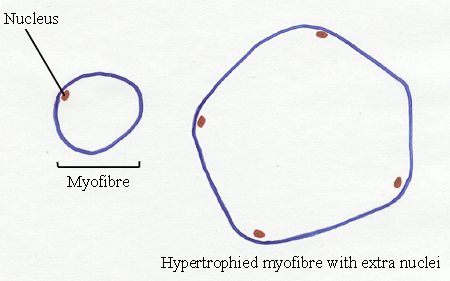
- It is important to remember when we look at
transverse sections of myofibres - the number of nuclei seen under the
sarcolemma (the cell membrane) depends on the thickness of the
transverse section relative to the length of the nuclei. If the
nuclei are long and thin, then they are more likely to be seen in
transverse section than short, thick nuclei. The thicker a
transverse section, the more nuclei will be seen.
- Even taking this into account, however, there is no
doubt the number of nuclei increases as myofibres undergo radial
hypertrophy.
- Where do the extra nuclei come from? This was a
puzzle for a hundred years. New nuclei are normally obtained from
mitosis (cell division),
and mitosis is normally detectable when chromosomes separate at metaphase - looking rather like
bunches of bananas. But, in a hundred years, nobody ever saw any
metaphase chromosomes in a myofibre.
Myofibre nuclei have the following features.
-
(1) No clear zone separates them from myofibrils.
-
(2) They are more basophilic (stained by basic dyes for
light microscopy) than fibroblast nuclei.
-
(3) The nucleolus (containing RNA) is well defined and
large.
-
(4) The outer chromatin (the granular pattern of stained
DNA) is
tightly distributed along the nuclear membrane.
-
(5) The internal cromatin is evenly dispersed.
Myofibre nuclei contain
DNA combined with histones and other structural
proteins to form chromatin.
When DNA is used for protein
synthesis, the chromatin is dispersed,
only binds weakly to
histological stains, and is called euchromatin. In non‑dividing
cells, chromatin may form darkly stained irregular clumps called
chromatin
particles. Nuclei also contain RNA and darkly stained clumps of RNA
form
nucleoli. The number of nucleoli may vary between animal species.
Condensed
regions of darkly stained chromosomes sometimes persist between cell
divisions
and are called heterochromatin. In the mononucleated cells of the body,
such as
those of the skin or liver, darkly stained chromosomes composed of
inactive DNA
are seen when cells divide. But, as explained below, the situation in
multinucleated myofibres is more complex, and distinct chromosomes
are not seen by light microscopy within myofibres.
On
or near the myofibre membrane are several types of nuclei.
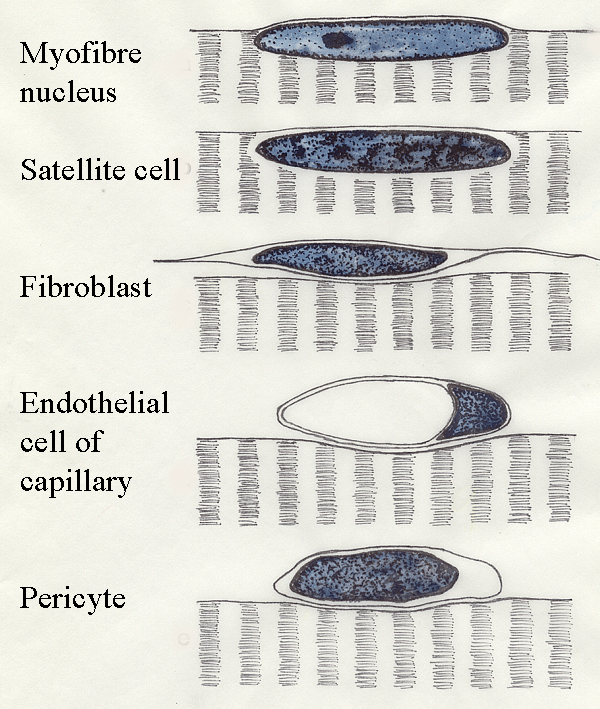
- True myofibre nuclei are located
within the sarcoplasm.
- Although the nuclei of
satellite cells are seen by
light microscopy within the sarcoplasm, electron microscopy shows satellite cells are
located in depressions in the myofibre surface.
- Thus, the nucleus of a
satellite cell
is separated from the sarcoplasm of its myofibre by a satellite cell
membrane and a myofibre membrane.

The red line above is where the TWO membranes are located - one around
the satellite cell and one lining the depression on the myofibre
surface.
The existence of satellite
cells became accepted in 1961, although they had been seen a hundred
years earlier - but no one believed it.
Pericytes
are mesodermal cells found around very small blood vessels. They
contain
actomyosin and are probably capable of contraction and phagocytosis.
The features of satellite cells are:
- (1) They are indented into a myofibre
surface, although sometimes they bulge outwards.
- (2) They have variable amounts of basophilic cytoplasm - usually
very little.
- (3) Their outer chromatin is heavily and unevenly
deposited along the nuclear
membrane.
- (4) Their internal chromatin is scattered in clumps.
- (5) Their nucleolus is small and
usually masked by internal chromatin - because
mitosis is still possible.
- (6) Sometimes the nuclei are uniformly stained dark.
- (7) Most of them have a clear space of 0.5 to 0.2
micrometres
separating them from the myofibrils - this
is where the two membranes are located.
- Muscle nuclei
increase in
number during postnatal development but the relative magnitude of the
increase
varies from muscle to muscle.
- Before the existence of satellite
cells was
proved by electron microscopy, it was rather difficult to explain how
muscle
nuclei were able to increase in number without any evidence, by light
microscopy, of mitosis in myofibre nuclei.
- Myofibre nuclei are
able to
withstand longitudinal compression during muscle contraction by means
of
concertina‑like wrinkles in their nuclear membrane and, for many years,
histologists regarded wrinkled muscle nuclei as evidence of an unusual
type of nuclear
division. They were wrong!
- New nuclei added
during the
postnatal growth of myofibres come from the daughter cells of
satellite
cell mitosis.
- Like myoblasts, satellite cells are part of the myogenic
cell
lineage from somitic mesoderm - essentially - they are premyoblasts which have been
trapped on the myofibre surface by the development of the endomysium (the
connective tissues around each myofibre).
- Mitosis in satellite cells has been seen by
electron microscopy
and the
synthesis of DNA by satellite cell nuclei has been proved by
radioautography (tritiated thymidine used in the synthesis of new DNA
is detected by its radioactivity).
- If a myofibre is damaged by disease, its myofibrils are
removed by phagocytic cells - then a new myofibre may be formed from
satellite cells within the old endomysial tube.
- There is some evidence satellite cells can move around on
the myofibre surface. Can you think how this might be exploited
agriculturally?
- On a proportional
basis, there
are more nuclei in red muscles than in white muscles, and
nuclei
are more frequent at the ends of myofibres than at their midlength.
- Younger animals have proportionally more satellite cells
than older animals.
The main two
functions of
satellite cells are to provide nuclei for growing myofibres and a
source of
myoblasts for postnatal muscle regeneration. These two functions may be
independently regulated by factors such as
insulin
and IGF (insulin-like growth factor) controlling the growth function,
and fibroblast growth
factor
controlling the regenerative function .
Why were mitotic chromosomes never observed inside myofibres ?
You would expect them if satellite cells look like other nuclei inside the
myofibre. The answer - satellite cells are too small to allow the
chromosomes to separate sufficiently to be seen clearly by light
microscopy.
Further information
Structure and Development of Meat
Animals and Poultry. Pages 389-396.






