15 Meat Colour
15.1 Introduction
Meat colour is
extremely
important when meat is presented for sale. If it does not look
right,
nobody will buy it. The subject divides naturally into two topics.
1. Light
scattering.
This determines whether meat is pale or dark.
2. Pigmentation. This determines whether the
meat
is purple (when first slaughtered), red (after exposure to air) or
brown
(after exposure to air for too long).
We
will also consider very briefly,
3. Colourimetry. Methods for measuring meat
colour
used industrially.
15.2 Light scattering and pH
Meat
with a low pH is generally more pale than at a high pH.
Paleness
related to pH is caused by light scattering.
Myofibrils
are a primary cause of pH-related light scattering in meat, but light
scattering
is also related inversely to
sarcomere
length.
We
do not yet know the relative importance of surface reflectance
from myofibrils versus refraction
through the depth
of myofibrils.
Precipitation
of sarcoplasmic proteins is
added to myofibrillar scattering when
pH is extremely low, or when pH reaches low levels while meat is still
hot.
Scattering
tends to decrease the length of the light path through meat. This
reduces selective absorbance by myoglobin. Thus, the colour
of meat is more conspicuous when pH is high.
15.3
Translucency of meat
Light entering meat is scattered by its complex microstructure. Scattering
may occur byreflection and
refraction, and may involve elastic
scattering (like Rayleigh
scattering in a blue sky). Meat has a complex microstructure,
with
curved membranes in various stages of disruption, myofilaments in
various
orientations and degrees of overlapping, and fluid compartments
changing
in refractive index.Scattering
tends to randomize the original directionality of the light. Light
escaping from meat becomes visible to the observer, while the remainder
is lost deep into the meat.
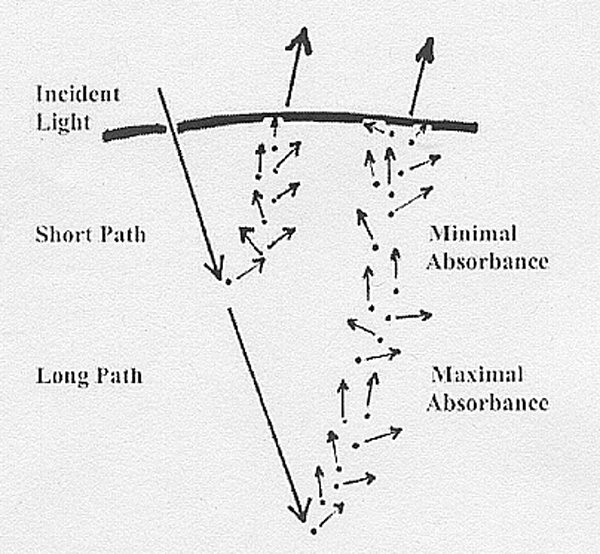
As
shown above, high
scattering causes a short
path length with minimal absorbance while
low scattering allows a long
path length with maximum absorbance by chromophores.
A chromophore (colour-bearer) is a coloured pigment.
15.4 Redness
- The
dominant pigment of meat is myoglobin.
- It
is dissolved uniformly through the sarcoplasm of myofibres,
particularly
those having strong aerobic metabolism
in the live animal.
- After
slaughter, myoglobin may move into the intercellular
space and may be lost in fluid dripping from the meat surface.
- Myoglobin
strongly absorbs green light.
- Thus,
light escaping
from
meat is dominated by yellow,
orange
and red, depending on the concentration of myoglobin.
- In
living muscle, myoglobin without much oxygen has a purple colour.
When myoglobin is oxygenated
(carrying
oxygen but NOT oxidized), it is bright red. Most
consumers
prefer their meat to be bright red.
- After
long exposure to oxygen, myoglobin becomes oxidized (a
chemical change) and is brown. Gourmet cooks prefer meat with a
brown
colour because it is likely to have a better taste than bright red
meat.
Strong
scattering shortens the light path through the meat, which reduces
selective
absorbance, and the observer sees a minimal myoglobin
effect.Conversely,
weak scattering allows a long light path and myoglobin becomes fully
visible.Metmyoglobin
(brown oxidized form of myoglobin) formation starts in subsurface
layers of the meat, and whether or not the observer detects its
brownness
depends on the degree of scattering and the depth of light penetration.
15.5 Sources of scattering
Extreme
PSE pork contains denatured
sarcoplasmic
proteins deposited on myofibrils. Denatured
proteins increase scattering and contribute to the paleness, but this
cannot
be the whole story, because dark-cutting beef scatters much less light
than normal beef, yet normal beef does not contain massive precipitates
of sarcoplasmic proteins. Protein precipitation is important, but only
in explaining extreme paleness, not the ordinary post-mortem
development
of meat paleness.
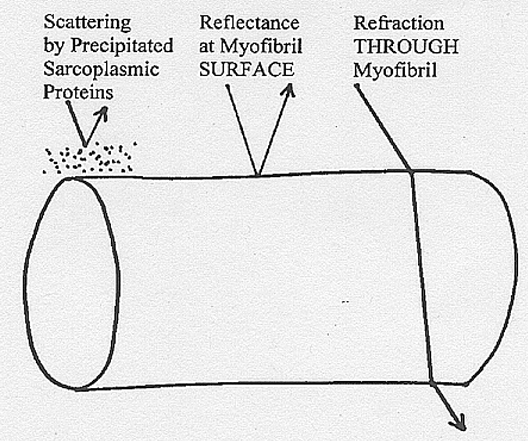
Scattering
from precipitated sarcoplasmic proteins, reflectance from the
myofibrillar
surface, and refraction through myofibrils all contribute to meat
paleness,
as shown above.
- Scattering
originates at boundaries between the sarcoplasm and the myofibrils.
- Although
the sarcoplasm of living muscle may have a higher refractive index than
the A band, and the A band may have a higher
RI than the I band, the myofilament lattice shrinks when pH decreases.
- Thus,
the myofibrillar refractive index increases as pH decreases, eventually
exceeding that of the sarcoplasm, and this increases light
scattering.
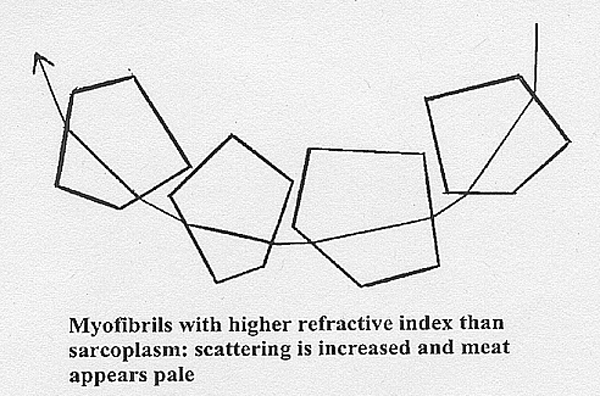
The
diagram aboveshows
how both scattering from precipitated sarcoplasmic proteins and
reflectance
from myofibrillar surfaces immediately scatters light back to the meat
surface to be perceived as paleness. But it may not be immediately
obvious
how refraction does the same thing. Light not reflected from the
myofibrillar
surface must enter the myofibril. If the myofibril has a higher
refractive
index than the sarcoplasm, the incident ray will be refracted towards
the
normal, and vice versa when leaving the myofibril. Thus, having
traversed a few myofibrils with a high refractive index, the light may
be scattered back to the meat surface to be perceived as paleness, as
shown
in the diagram below. When
sarcoplasm and myofibrils have similar refractive indices, the incident
illumination penetrates deeply into the meat, which then appears dark
and
strongly pigmented.
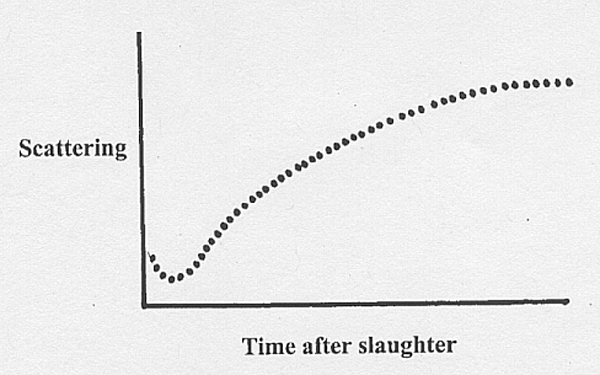
15.6 Post mortem changes in scattering
- Sometimes we can detect an early
post mortem decrease
in scattering, perhaps reaching a minimum when sarcoplasm and
myofibrils
have the same refractive index.
- But this
transient effect might also originate at the boundary between
the
intercellular fluid and the plasma membrane of the myofibre, because
increased
osmotic pressure from glycolysis within the myofibre may cause a
transient
uptake of intercellular fluid.
- This uncertainty results from
the
difficulty
of making optical measurements on the muscles of meat animals as they
are
being slaughtered, dressed and refrigerated. But there is no doubt
scattering
eventually increases after slaughter, unless a carcass is to remain as
a dark-cutter because of minimal glycogen, reduced glycolysis and a
high
ultimate pH .
Changes
in scattering after slaughter - sometimes a little decrease at first,
but
always a large increase later (except if the meat is dark-cutting or
DDD
when it does not increase).
15.7
Derivatives of myoglobin
- The
familiar colours of meat are the purple
colour of myoglobin, the bright
red colour of oxymyoglobin, and the brown
colour of metmyoglobin.<>
- <>Dietary supplements of vitamin E retard
the
formation
of metmyoglobin.
- Green pigments caused by the formation of sulphmyoglobin
are rarely seen in the normal course of meat handling. Sulphmyoglobin
may
be formed if bacteria are able to produce hydrogen sulphide which then
acts on myoglobin.
Myoglobin
derivatives differ in their absorbance and reflectance spectra and the
ratio of measurements at two different wavelengths may be used to
calculate
the relative amounts of myoglobin derivatives. An
isobestic point occurs when two or more spectra intersect
to
give the same value at the same wavelength. An isobestic point for
myoglobin,
oxymyoglobin and metmyoglobin is at a wavelength of 525
nm. An absorbance peak for metmyoglobin is at
630 nm. Thus, the ratio of measurements at 630 nm (mostly
metmyoglobin)
to measurements at 525 nm (sum of all three myoglobins) contains
information
on the amount of metmyoglobin as a fraction of the total myoglobins.
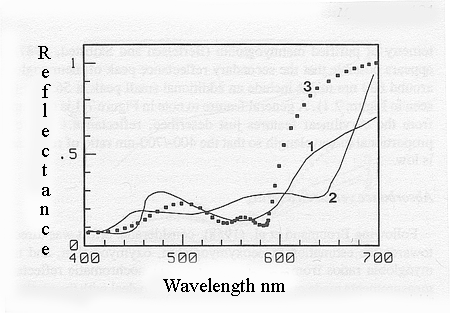
Reflectance spectra of myoglobin
(1),
metmyoglobin (2) and oxymyoglobin (3).
The
strong absorbance bands that occur at low visible wavelengths are
called Soret
absorbance bands. The Soret absorbance bands for myoglobin,
oxymyoglobin
and metmyoglobin are at 434, 416 and 410 nm, respectively. Myoglobin
has
an absorbance band at 555 nm that is replaced in oxymyoglobin by a
strong
absorbance band at 578 nm and a slightly weaker band at 542 nm. In most
practical situations, however, the formation of oxymyoglobin in fresh meat
is accompanied by a trace of metmyoglobin formation so that these two
absorbance
bands (at 578 and 542 nm) are approximately equal.
15.8 Colourimetry
There
are three words with special meanings in relation to the human
perception
of colour.
- The
general type of colour, such as red, blue or green, is called a hue.
If, for the sake of explanation, red paint was mixed in increasing
quantities
into a pot of dull white paint, the paint would change through pale
pink
to dark red, yet the hue (red) is unchanged.
- The
property of colour which changes in this example of paint mixing is the
intensity of the colour, and this is called the chroma.
- If
the paint mixing example had started with bright white paint instead of
dull white, the final product would be brighter, and would have a
greater luminosity.
There
are several different systems for the measurement of colour in these
terms,
and the choice of which system to use depends largely on what equipment
is readily available. In
the method recommended by the International
Commission on Illumination (CIE), the
primary
hues, red, green and blue, are added or subtracted from each other to
match
any colour. By a mathematical manipulation, it is possible to specify
both
hue and chroma in the CIE system by means of a single pair of
chromaticity
coordinates called x and y.
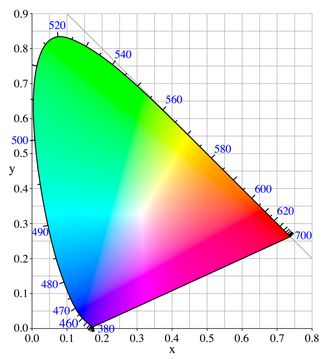
Changes in hue follow the contour shown from 380 to 700 nm in the
diagram
above, while changes in chroma radiate from the central position
of white. Luminosity is specified by a third coordinate relative to the
plane of the chromaticity coordinates. To illustrate this dimension the
diagram above of the chromaticity coordinates is shown
pinned
to a drawing board, and a pencil is held perpendicular to the board.
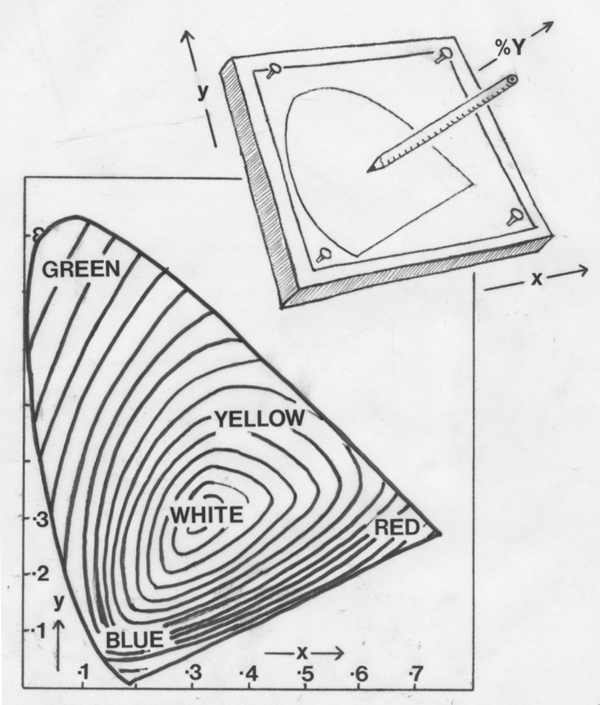
Luminosity is measured at a point along the pencil, and this
dimension
is called percent Y. In meat with a lot of marbling fat, it might be
expected
measurements made by reflectance spectrophotometry would be biassed by
light reflected from marbling fat. However, this source of error
appears
to be quite small in actual practice.
15.9 Meat colour problems
- Meat
processed with sodium nitrite develops a heat-stable pink colouration
from dinitrosylhaemochrome.
This is the attractive pink colour of ham.
- However,
formation of this or similar pigments when they are not wanted can
create
problems. Meat retaining a pink colour after extensive cooking
causes
consumers to suspect the meat has not been properly cooked. Nitric
oxide and carbon monoxide
from oven gases may be involved in forming heat?stable pink derivatives
of myoglobin. Similar problems may be associated with high nitrite
levels
in the feed, treatment with sodium erythorbate, irradiation and
freezing.
Further information
Swatland,
H.J. 2004. Progress in understanding the paleness of meat with a low
pH.
South African Journal of Animal Science, 34: 1-7.