
If a small chunk of meat is placed under a dissecting microscope and teased apart with needles, the smallest fasciculi visible without magnification are composed of bundles of myofibres. Myofibres are the basic cellular units of living muscle and of meat. They are unusual cells because they are multinucleate (with many cell nuclei) and are extremely long (commonly several centimetres) relative to their microscopic diameter (usually less than one tenth of a millimetre).
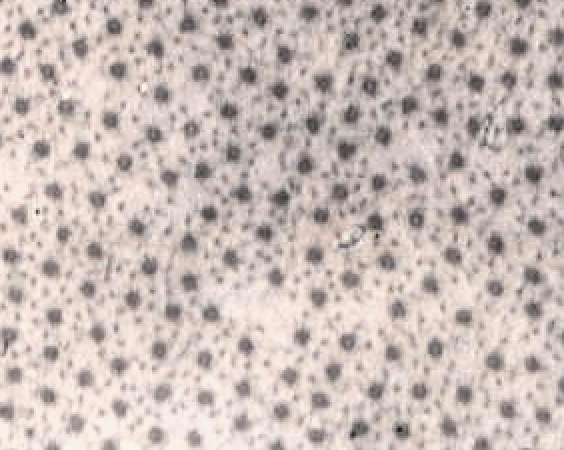
The image below shows a short part of one muscle fibre,
but even this contains many nuclei (the dark blobs).
The myofibres found in most commercial cuts of meat seldom
run the
complete length of the muscle in which they are located. Individual
fibres within a fasciculus may terminate at a point along the length of
the fasciculus at a tapered ending anchored in the connective tissue on
the surface of an adjacent myofibre so that tapered endings transmit
their
force of contraction to the endomysium
(the connective tissue around each myofibre).

.
Apart from tapered intrafascicular endings, the diameter
of
a myofibre is assumed to be approximately constant along its length.
Myofibre
diameters slowly increase during the growth of a muscle (radial hypertrophy), but they also
increase temporarily when a myofibre contracts. Thus, when measuring
myofibre
diameters in a growth study, special care must be taken to avoid or to
correct for differences in the degree of muscle contraction.
Place some meat fragments together with some water in a kitchen blender. After running the blender for a few seconds, the connective tissue holding the myofibres together is disrupted to leave a pale red suspension of broken myofibres in water. The red colour comes from myoglobin, the soluble red pigment from inside the myofibres. Place a drop of the macerated muscle suspension on a microscope slide beneath a cover slip, as shown in the image below where, at the bottom of the frame, is an intact myofibre and above it is a smashed myofibre with all its myofibrils visible.
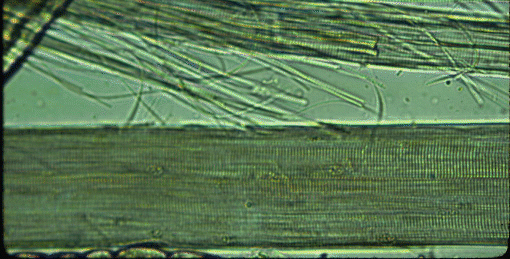
The transverse striations of myofibres become visible if the iris diaphragm of the sub stage condenser is almost closed (the gain in contrast is offset by a loss of resolution, which is why the diaphragm is normally open wider). With a high magnification microscope objective, myofibrils may be seen if they protrude from the broken end of a myofibre or if they have escaped from a broken myofibre. Under the surface membranes of myofibres may be seen some flattened bubble-like inclusions. These are the nuclei of the myofibre, and their DNA may be stained by treating the macerated muscle suspension with dyes such as haematoxylin. On the surfaces of any myofibres retaining some of their surrounding connective tissue may be seen branching capillaries, once part of the vascular bed of the muscle. Red blood cells (erythrocytes) are rarely seen in the capillaries.
When meat animals are slaughtered, they are shackled and suspended from their hindlimbs and some muscles, such as the filet or psoas muscles ventral to the vertebral column, become stretched. Other muscles, such as those in the posterior part of the hindlimb, are free from skeletal restraint and may contract weakly as the carcass becomes stiff after death (rigor mortis). If samples from stretched and contracted muscles are compared, transverse striations will appear relatively far apart in the stretched muscle and closer together in the contracted muscle.
The distance between the transverse striations is the sarcomere length.
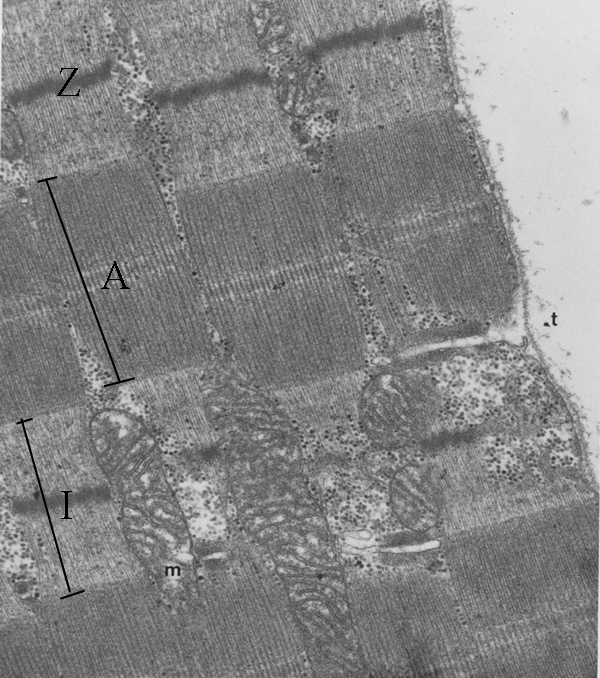
Other features of the fibril are
detectable by light microscopy under optimum conditions, but these
details are seen more clearly by electron microscopy. A thin Z line
or disc occurs at the middle of the I band. Also marked are some other
features - a mitochondrion (m) and the start of a transverse tubule (t)
which we will consider later. If the clear spaces at top right is
outside the myofibre, then you can see the membrane around the
myofibre, and you can see the transverse tubule is a finger-like
inpushing connecting with the space outside the myofibre.
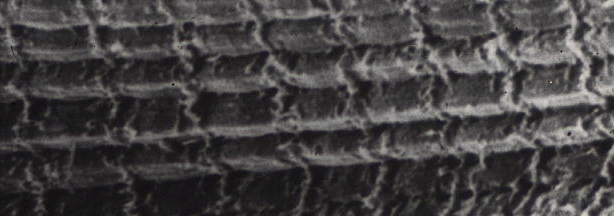
The transmission
electronmigrograph above is not the only way to look at transverse
striations. The image below is a scanning electron micrograph (showing
the surface of a specimen rather than looking through a thin slice of
it), and the myofibrils run horizontally across the image, showing
transverse striations vertically. Only, of course, they do not look the
same as they did in the previous image. In the scanning micrograph we
see the sarcomeres because of the raised region of the Z-line attached
to the cytoskeleton (desmin).
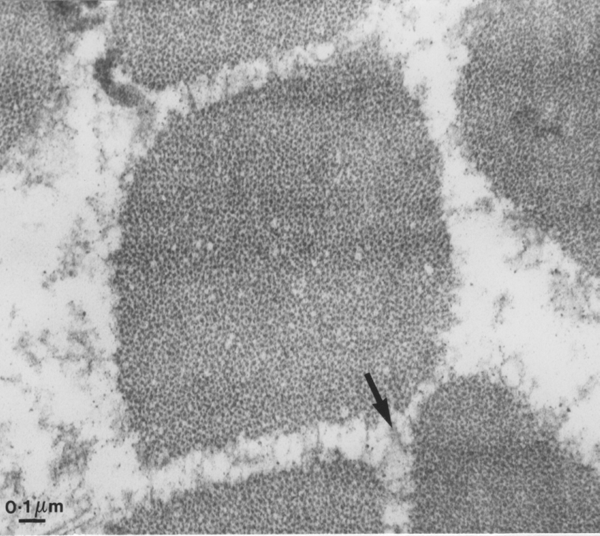
Yet another way of looking at myofibrils is to examine a transverse section by transmission electron microscopy, as seen below. We are looking inside one myofibre of pork and once myofibril is centred in the field. From the bar scale at the bottom left you can see the myofibril is a little over one micrometre in diameter. The space around the myofibril is greatly enlarged because the myofibril is loosing fluid - more about this multimillion dollar problem later in the course! The arrow shows part of the cytoskeleton resisting the shrinkage of the myofibrils and the loss of fluid from the myofibril.
.
When a myofibre contracts, the thick filaments slide between the thin filaments so the I band gets shorter. The length of the A band remains constant. This is called the sliding filament theory of muscle contraction proposed in 1972. If a muscle is at its resting length, the gap between opposing thin myofilaments at the mid-length of the sarcomere causes a pale H zone in the A band. Although the sliding myofilament theory now is widely accepted, there remain many unsolved problems in the mechanism of the system.
Contraction is an active process requiring energy, which is provided by the hydrolysis of phosphate from adenosine triphosphate (ATP), although the transduction from chemical to mechanical energy may be delayed until the resulting adenosine diphosphate (ADP) and inorganic phosphate are released by myosin when it recombines with actin. Myosin is the dominant protein of thick myofilaments. Actin is the dominant protein of thin myofilaments. Contraction by myofilament sliding may be achieved by the rowing action of numerous cross bridges protruding from the thick myofilaments, Cross bridges are formed from the heads of myosin molecules whose backbones are bound into the thick myofilament (more details later). However, the conformational change causing the cross bridge movement does not seem to be a simple angular change of the cross bridge as was originally supposed, and the movement probably originates elsewhere in the molecule, whose structure was first established three-dimensionally in 1993.
Myoilament sliding and muscle
contraction
come from the rowing action of very large numbers of myosin molecules.
Each
individual stroke by a myosin molecule head takes about 1 millisecond
and produces a 12 nm movement. Although this is a very small distance,
many thousands of sarcomeres are arranged in a series, and in a very
short time the sum of all these small distances may be measured in
centimetres. The myosin head only releases its grip on the actin, and
swings back for another power stroke with another actin, if it is
recharged with another ATP molecule. Thus, when muscles are converted
to meat and no more ATP is available,
thick and thin filaments lock
together wherever they overlap. This prevents any further
filament
sliding and the muscle becomes almost inextensible: this condition is
called rigor mortis. Rigor mortis is very important in the meat
industry - it is taken as the point at which muscle becomes meat.
This important topic will be considered in more detail later.