17 Cooking Meat
17.1 Why is meat cooking included in this course?
- Cooking meat is extremely important - although not many
animal scientists can see this. Animal scientists help farmers produce
meat. But there is no point in producing meat unless it can be sold.
Meat consumption depends on consumers knowing how to cook meat.
Educating our customers so they do not
ruin the quality of the fresh meat we sell them is extremely important
for the
future of the whole meat industry.
- Restaurants and fast-food outlets already have a legal
responsibility
for meat cooking and, with the general trend to make institutions
responsible
for things that once were the responsibility of the individual, the
burden may
one day be placed on the meat industry to make a demonstrable attempt
to ensure all meat is cooked appropriately by our customers.
- A preventive approach to problems tends to
be more effective than a retroactive salvage operation, and we should
now be
starting to educate more of our customers. What better place to
start than with you students?
- In the
domestic kitchen, many meat eaters already are overcooking their
meat.
If the trend continues, driven by a growing
concern with microbial contamination of meat, it will further increase
customer
dissatisfaction with prime cuts of meat, because they are expensive yet
toughened
by overcooking. Other pressures are pushing our customers in
this
direction as well, for example, PSE pork.
An exudate from PSE pork coagulating on the meat surface during cooking
encourages overcooking, until the meat surface looks dry, at which
point the
pork is overcooked and tough.
17.2 The basics
-
Cooking kills bacteria and parasites. Bacteria are
everywhere. Parasites are rare. Most of the bacteria are on the
surface of the meat and are soon killed by cooking unless the meat surface has been mixed
deep into the meat - HAMBURGERS MUST BE COOKED ALL THE WAY THROUGH!
- Cooking changes and strengthens the
taste and aroma of meat. Raw meat has a rather subtle taste -
consumers who like their steaks rare are really going for the
texture. The strongest taste is found on the outsides of roasts.
- Cooking changes the colour of meat -
typically from red to brown. Consumers who like rare meat will
want to see red, while those who like well-cooked meat will want to see
brown. But we are
all really using colour to guess what temperature the meat has reached
during cooking.
- Cooking changes the texture of
meat. The key point many
consumers are missing is - COOKING MAKES TENDER MEAT TOUGH
whereas COOKING MAKES TOUGH MEAT TENDER. Why?
17.3 Measuring temperature
Hopefully
there is no need to explain how ordinary thermometers work in the home,
but we use some other methods industrially.
- A thermocouple is
composed of two dissimilar electrical
conductors joined at their ends to generate a thermoelectric voltage directly proportional
to the temperature difference between the two end junctions.
Iron-constantan and chromel-alumel
thermocouples are the most common examples. Junctions may be exposed or
covered, and/or grounded.
- A thermistor
is a resistive circuit component, usually a two-terminal semiconductor, whose resistance decreases as temperature increases.
- A Callendar's thermometer (platinum
RTD probe) uses a thin film or wire of pure
platinum, which has a
relatively high resistance (for a metal), thus facilitating the
measurement of
the change in resistance with temperature. In metals, resistance increases as temperature increases.
- Microwave cooking is monitored using a quartz optical fibre with a fluorescent
tip. Typically, the fluorescence spectrum changes with the temperature
and is determined from optical measurements at two wavelengths.
- Everything emits infrared. The hotter the
meat, the more active its molecules are, and the more infrared
energy it emits. An infrared
thermometer detects this radiant energy
In
practice, things to watch out for are
the time constant, the length
of time the sensor takes to read the true
temperature of the sample, and heat
conduction along the sensor, its wire
connections, and its protective shielding.
For example, when monitoring the cooking of a roast, an unsuitable
temperature probe may pick up heat from the air in the oven and conduct
it into
the roast faster than the heat can move through the meat. Thus, colour
changes
in the interior of the roast may take place first along the route of
the
temperature probe.
17.4 Colour changes
Meat with an appreciable myoglobin concentration changes from red
to grey or
greyish-brown when cooked. The brown pigments formed during
cooking may include
- denatured
globin nicotinamide hemichromes,
- denatured myoglobin,
- Maillard reaction products,
- metmyochromogen and
- haematin di-imadazole complexes.
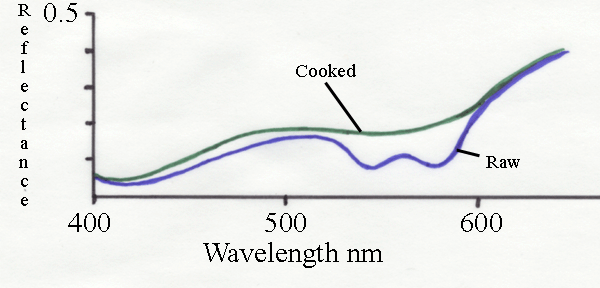
The spectrum above shows the change in reflectance as beef is
cooked. Raw beef has a characteristic dimple around 560 nm which
indicates myoglobin. Much of the green light is absorbed and the
meat appears red. After cooking, there is a large increase in the
reflectance of green light. This is the major cause of the meat
appearing brown when cooked.
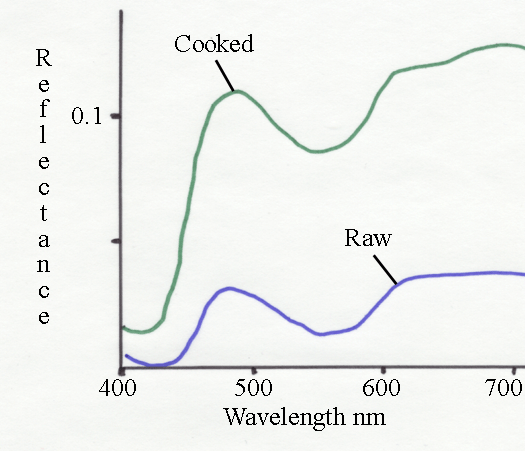
Something else occurs, as shown in the spectrum above for the cooking
of lamb. The reflectance units are not directly comparable (they depend
on the apparatus and how it is standardized against a 100% white
object). Also, the myoglobin dimple is missing in the raw sample -
because metmyoglobin formation has started. The major change
between raw and cooked lamb is a massive increase in reflectance at all
wavelengths - thus, the cooked lamb is scattering more light than the
raw meat. In the CIE system this appears as a large increase in
%Y. This increase in scattering is clearly visible because the
myoglobin level is lower in lamb than in beef. What happens when egg
albumen is cooked?
Colour changes during cooking are important for a variety of
reasons. Cooking protect us
against trichinosis and potentially deadly bacteria, but overcooking
beyond the
safe point may render meat unpalatable.
In most practical situations the safe point is judged not by
temperature,
but by colour, which is why failure to attain a cooked meat colour even
after
reaching a safe temperature is a problem - partcularly in poultry meat.
There are numerous reasons proper cooking of meat may fail to produce a
brown colour. Nitrites act on myoglobin to form a heat-stable pink
pigment (often call dinitrosylhaemochrome).
Nitrites are sometimes found in high levels in well-water and spices
(for example, in dried celery powder). Carbon monoxide forms a
heat-stable pink pigment from myoglobin. Animal exposed to
vehicle exhaust gases during transport may produce meat which will not
brown properly when cooked. When cooking, gas ovens may produce nitrous
oxides leading to surface
formation of dinitrosylhaemochrome. The surface of meat cooked with
bacon (residual nitrites present) may fail to brown properly.
17.5 Effect of cooking on collagen
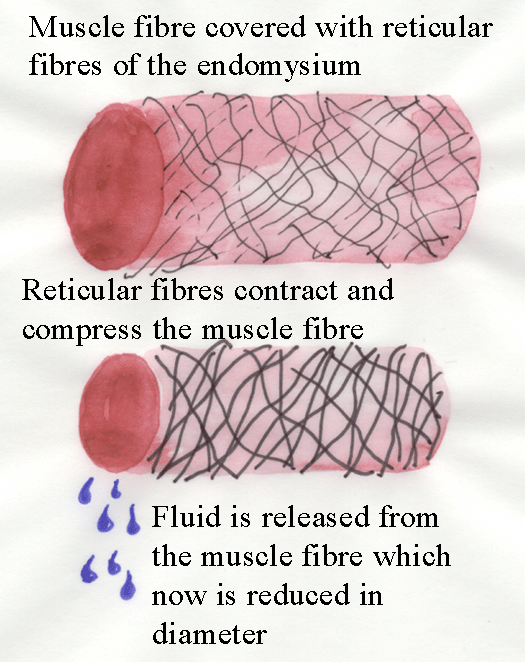
Cooking has major effects on the connective tissue of meat.
Firstly, cooking causes collagen fibres to contract (this decrease in length has NO
connection to muscle fibre contraction!).
The diagram below explains why fluid is released from meat as it
is cooked.
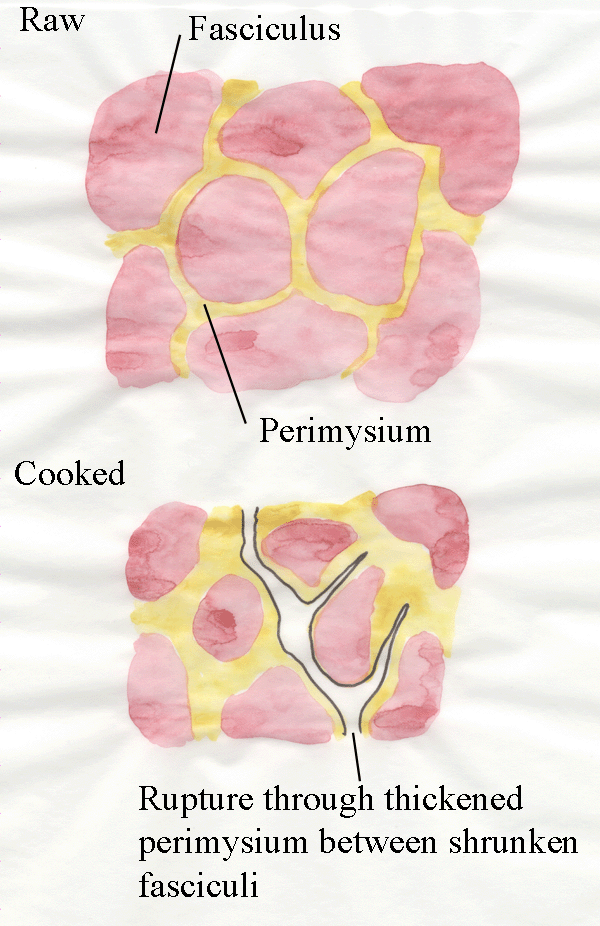
Secondly, further cooking causes the gelatinization of collagen fibres.
In other words, collagen fibres are converted from structures like
miniature steel cables to jelly.
The diagram below explains the weakest point through the
connective tissue of cooked meat.
The key point? Cuts of meat with a high connective tissue content
must be cooked with moist heat for an extended time - for
example, stewed or casseroled. This takes time and effort -
so the meat is less expensive than cuts with a low connective tissue
content which can be barbecued. However, cuts of meat with a high
connective tissue content usually have an excellent taste. If they are
put through a meat grinder (technical term - ), the connective tissue
toughness
is cancelled and, as you all know, hamburgers can be barbecued! So, in
the summer, tough beef gets used for hamburger while, in the winter,
more of the tough beef is sold intact for stewing.
The bright, enquiring mind of a good student might ask at what
temperatures do collagen contraction and gelatinization occur? Are
there breed effects? Which breed has the lowest gelatinization
temperature? Are there nutritional effects? What feeding causes
the lowest collagen gelatinization temperatures? How does animal age
affect all this? Sadly, because animal scientists tend to ignore
cooking, and food scientists tend to ignore animal science - we do not
know. Observations on the Swatland family Sunday roast show
considerable variability. Contraction starts before
gelatinization, and both are somewhere between 60 and 70º C.
Despite the millions spent on agricultural research across Canada, it
is no wonder we all get annoyed by tough meat now and again!
17.6 Effect of cooking on myofibrils
Cooking makes
myofibrils stronger. The longer we cook myofibrils, the tougher
they get. Cooking cross-links the proteins. If sarcomere
length is short, there is more cross-linking and cooked sracomeres are
very strong - TOUGH MEAT. So, take a steak like beef filet with a very
low connective tissue content. The longer you cook it, the
tougher it gets. Take a steak like a cross-cut arm roast of beef
with quite a high connective tissue content. Cooking makes all
the myofibrils tougher, but reduces the strength of connective
tissue. Thus, the overall effect is - the longer the cooking, the
more tender the meat.
17.7 Objective measurement of meat toughness
The best method of quantify meat toughness is sensory
evaluation. Cooked meat is tested under standardized conditions
by typical consumers or a trained group of experts. The results
are obtained by asking questions - rate the meat tenderness on a scale
of, say, from -5 (very tough), through 0 (undecided), to +5 (very
tender). But this is a lengthy and expensive process. Objective methods
are used more commonly in the meat industry.

Method A. The cooked meat
sample is compressed between two blades. Blunt blades are preferred
because sharp blades cannot be kept in a constant state of sharpness.
Method B. The is the most
commonly used method. It is called the Warner-Bratzler test after
the two scientists who first invented it many years ago. The meat
sample is restrained in the triangular opening (with squared edges) of
a metal blade.
Method C. Needles are pushed
into the meat.
Method D. The force needed to
push the meat through a meat grinder is found electrically.
Methods E and F. The
tensile strength of the meat is measured - but it is difficult to get
hold of the sample without altering its properties. Glue is used
in method F.
Meat tenderness is studied by rheology
(the study of flowing systems, whether they be meat or concrete). The
forces used to rupture the meat are measured in Newtons. Although often called shear
tests - this is incorrect. Shearing only occurs in rigid
materials. When meat fails, either between your teeth or in
rheological apparatus, the ultimate failure is the tensile strength of
either connective tissues or myofibrils.
Further information
Structure and Development of Meat
Animals and Poultry. Pages 255-269.




